-
- The table below gives information about some oxides. Study the information and use it to answer the questions that follow.
Formula of oxide
Melting point
Effect of adding water to oxide
Effect of electric current on molten oxide
Effect of adding aqueous sodium hydroxide to oxide
Na2O
920
Dissolves readily
Conducts; Na (s) and O2 (g) produced
(a)
P2O5
563
Dissolves readily
(b)
(c)
SO3
17
Dissolves readily
Does not conduct
Reacts readily
Al2O3
2045
Does not dissolve readily
(d)
Reacts readily
- Write the missing information for spaces a to d (2mks)
- Write equations for the reactions that take place between
- SO3 gas and water (1mk)
- SO3 gas and sodium hydroxide (1mk)
- Why is it not advisable to carry out reaction ii (a) above in the laboratory? (1mk
- Explain the difference in the melting points of P2O5 and SO3 (1mk
- Phosphorous (V) oxide dissolves in water to form phosphoric acid. State and explain how the ability of concentrated phosphoric acid to conduct electricity compares to that of dilute phosphoric acid (2mks)
- During the industrial manufacture of hydrochloric acid, hydrogen gas and chlorine gas are the raw materials.
- Write an equation for the reaction that occurs between hydrogen and chlorine in the burning chamber (1mk)
- What is the purpose of the glass beads in the absorption chamber after the reaction between chlorine and hydrogen? (1mk
- Given that the percentage purity of the hydrochloric acid manufactured is 35% and its density is 1.18g/cm3 determine the concentration of the acid. (H=1, Cl=35.5) (2mks
- The table below gives information about some oxides. Study the information and use it to answer the questions that follow.
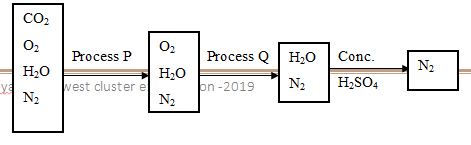
- The flow diagram below shows the process of obtaining nitrogen by fractional distillation. Use it to answer the following questions.
- What is the purpose of the processes P and Q. (2 Marks)
- Identify the reagents used in processes P and Q (2 Marks)
- Write equations for the chemical processes in P and Q (2Marks)
- Give four uses of oxygen (2 Marks)
- Give an impurity that could be found in the nitrogen gas obtained above. (1 Mark
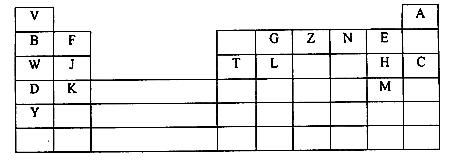
- The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements
- What name is given to the family of the;
- Elements to which E, H and M belong? (1mk)
- Elements to which F, J and K belong? (1mk)
- Write the chemical formula of the;
- Sulphate of T. (1mk)
- Nitrate of J. (1mk)
- Name the type of bond and structure formed between reactions of:
- D and N. (1mk)
Bond
Structure - T and H. (1mk)
Bond
Structure
- D and N. (1mk)
-
- Ionic radius of element E is bigger than its atomic radius. Explain. (2mks)
- The Oxide of G has a lower melting point than the Oxide of L Explain. (1mks)
- Explain in terms of bonding and structure the following observation. There is an increase in melting and boiling points from W to T.(2mks)
- Using dot (∙) and cross (X) diagram show bonding in ZV+4. (2mks)
- What name is given to the family of the;
- Below is a brief outline of a method used in preparing lead chloride. Lead carbonate is added to warm dilute nitric (V) acid. When the carbonate has reacted with the warm acid, more carbonate is added until the carbonate is in excess. The mixture is filtered. Some sodium chloride solution is added to the filtrate.
-
- What observations are made when lead carbonate is added to the warm nitric (V) acid. ( 1 mark)
- What happens when sodium chloride is added to the filtrate? (1 mark)
- Write an equation for the reaction between:
- The carbonate and the acid. (1 ½ marks)
- The filtrate and sodium chloride (1 ½ marks)
- Write an ionic equation for the reaction in b (ii) (1 marks)
- Name the reaction that takes place between the filtrate and sodium chloride.(1mark)
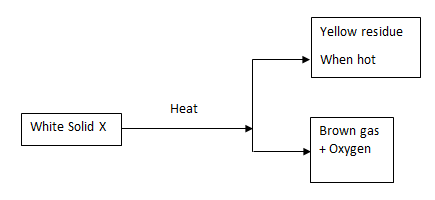
- Study the scheme below and answer the questions that follow.
Name- Solid X (1 mark)
- The yellow residue (1 mark)
- Write an equation for the decomposition of solid X. (1 mark)
-
- At 25°C 50g of substance X were added to 100g of water to make a saturated solution.
- What is meant a saturated solution? (1mk
- The table below gives the solubilities of substance X at different temperatures.
Temperature °C
14
24
33
40
46
52
Solubility g/100g H2O
24
36
50
62
72
90
- Plot a graph of the solubility of substance X (vertical axis) against temperature. (3mks
- Using the graph.
- determine the solubility of substance X at 20°C. (2mks)
- determine the mass of substance X that remained undissolved given that 90g of substance X were added to 100cm³ of water and warmed to 35°C. (2mks)
- Calculate the molarity of the solution at 30°C. (Relative formula mass of X = 122.5). (3mks)
- In an experiment, soap solution was added to three separate samples of water. The table below shows volumes of soap solution required is form lather with 1000cm³ of each sample of water before and after boiling.
Sample
1
2
3
Volume of soap before water is boiled (cm³)
25.0
5.0
10.0
Volume of soap after water is boiled (cm³
25.0
5.0
5.0
- Which water was likely to be soft? Explain. (2marks)
- Explain the change in volume of soap solution used in sample III. (1mark
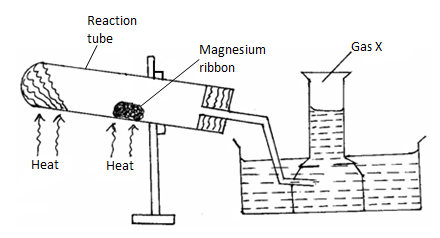
- The set-up below was used to prepare and collect gas X. During the experiment cleaned magnesium ribbon was strongly heated before heating the wet glass wool.
- Why is magnesium ribbon cleaned before it is used? (1 mark)
- Name gas X(1 mark)
- State one observation that would be noted in the reaction tube. (1 mark)
- Write the equation for the reaction in the reaction tube. (1 mark)
- State one industrial use of the solid product formed in the reaction tube. (1 mark)
- What precaution should be taken at the end of experiment? Explain. (2 marks)
- At the end of the experiment 96.0cm³ of gas X were collected at 10°C and 1 atmosphere pressure. (Mg = 24, M.G.V = 22.4, T = O°C at stp, P = 1 atmosphere at stp).
- Determine the volume gas X would occupy at s.t.p? (2 marks)
- Calculate the mass of magnesium ribbon used Mg = 24. (2 marks
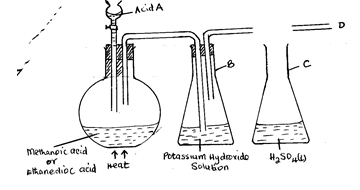
- The set-up below was used in a chemistry laboratory to prepare and study the properties of carbon(II) oxide . Use it to answer the questions that follows.
- Name acid A
- State the purpose of potassium hydroxide solution (KOH) in the set-up (1mark)
-
- Complete the diagram to show how carbon (II) oxide would be dried by concentrated sulphuric (VI) acid. (1 mark)
- Explain why concentrated sulphuric (VI) acid is a suitable drying agent for carbon (II) oxide. (1mark)
-
- From point “D” in the diagram, draw a well labeled diagram in the space provided below to show how the reducing properties of carbon(II) oxide on copper (II) oxide could be studied. (3mark
- Write an equation to show the reduction of copper (II) oxide by carbon (II) oxide. (1 mark
- Identify the gaseous product from the reaction in d(ii) above (1mark)
- State how the gas named in d (iii) above can be tested (2marks)
Download CHEMISTRY PAPER 2 - KCSE 2019 NYANDARUA PRE MOCK EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students