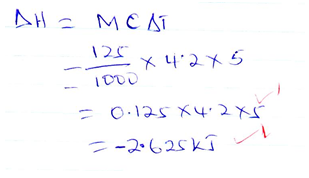QUESTIONS
-
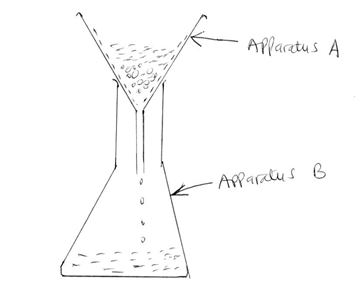
- The diagram below shows a set-up used to separate solid K and liquid R
- What name is given to the method of separation shown above [1mk]
- What physical property of solid K makes it possible to separate from the mixture as shown above [1mk]
- State one example of a mixture that can be separated as shown above [2mks]
- Name one other method that can be used to separate the mixture of K and L [1mk]
- Identify apparatus A and B [2mks]
- State two other possible uses of apparatus A, other than the one shown on the diagram [2mks]
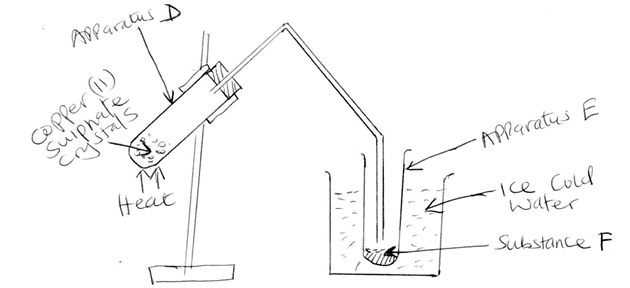
- The diagram below shows part of a set-up used to investigate the effect of heating copper(II) sulphate crystals
- State two likely observations made in apparatus D [2mks]
- Identify substance F [1mk]
- Was the change undergone by the copper {II} sulphate crystals temporary chemical change or permanent chemical change? Explain your answer [2mks]
- The diagram below shows a set-up used to separate solid K and liquid R
- In an experiment to determine the enthalpy of displacement when zinc is heated with copper (II)Sulphate, excess zinc powder was added to 125cm3 of 0.75M copper {II} Sulphate solution in a beaker. The temperature of the mixture changed from 240Cto 290C. Assuming that the density of the solution was 1g/cm3 and its specific heat capacity was 4.2KJK-1K
- Write a chemical equation of the reaction that occurred [1mk]
- Why was it necessary to use excess zinc powder [1mk]
- State and explain one observation made by the end of the experiment [2mks]
- Determine the enthalpy change in the reaction that occurred [3mks]
- Determine the molar enthalpy of displacement of copper (II) ions by zinc [3mks]
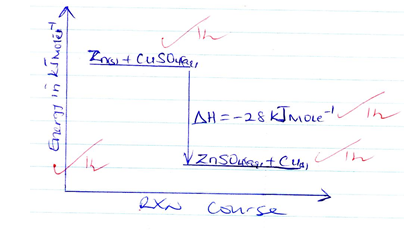
- Represent the reaction on an energy level diagram [2mks]
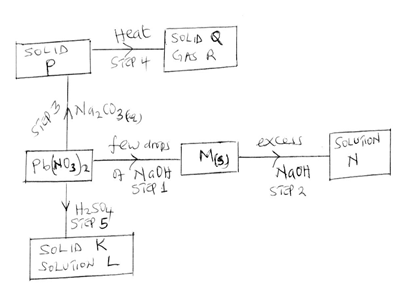
- Study the diagram below and answer the questions that follow;
- Identify the following [4mks]
- Substance M
- Solid P
- Gas R
- Solution L
- Write the ionic equation of the reaction occurring at step
- Step 2 [1mk]
- Step 3
-
- State the observation made when ammonia solution is added to lead {II} nitrate solution drop wise till excess [2mks]
- The equation below shows a reversible reaction
H3O+(aq) + HSO4- (aq) →H2O[l] + H2SO4[aq]
- Identify the acid in the fowards reaction [1mk]
- Identify the base in the reverse reaction [1mk]
- Identify the following [4mks]
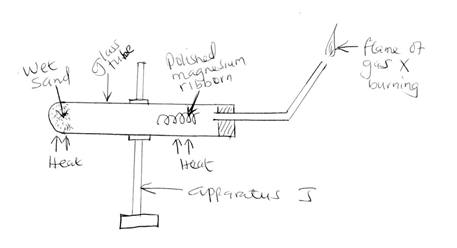
- The diagram below shows a set-up used to investigate the reaction of magnesium and steam
- Identify gas X [1mk]
- Write a chemical equation of the reaction;
- Occurring in the glass tube [1mk]
- Leading to the flame of the burning gas X [1mk]
- Explain why it was necessary to;
- Polish the magnesium ribbon [1mk]
- Heat the wet sand before heating the magnesium ribbon [1mk]
- Identify apparatus J [1mk]
- How does the mass of the magnesium compare before and after the experiment. Explain.[2mks]
- State two physical properties of gas X [2mks]
- State two uses of gas X [2mks]
- State the role of the wet sand [1mk]
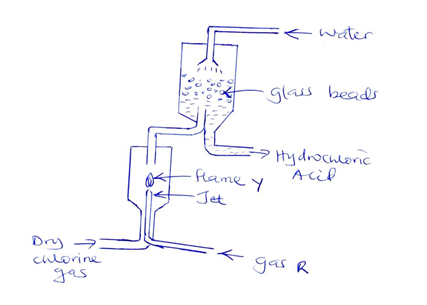
- The diagram below shows a flow chart representing process used in the industrial manufacture of hydrochloric acid
- Identify gas R [1mk]
- Write a chemical equation for the reaction between gas R and chlorine gas [1mk]
- State one possible role of the glass beads [1mk]
- State one possible source of;
- Gas R [1mk]
- Chlorine gas [1mk]
- Explain why it’s necessary to allow only small amount of gas R to burn in excess chlorine gas in the above process [1mk]
- State two uses of hydrochloric acid [2mks]
- Describe a confirmatory test for hydrogen chloride gas [2mks]
-
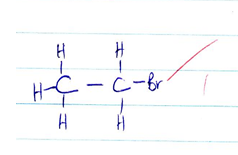
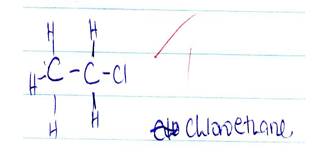
- Name the following hydrocarbon [3mks]
- CH3CH2CH3
- CH3CHCHCH3
- CH3CHCHCH2CH2Br
- Study the reaction below;
CH2CH2 + HBr → substance T- Name substance T [1mk]
- Draw the structural formulae of substance T [1mk]
- Draw the structural formulae and name the compound that would be formed if chlorine gas was used in place of HBr [1mk]
- State one domestic use of ethane [1mk]
- State two natural sources of alkanes [2mks]
- Name the following hydrocarbon [3mks]
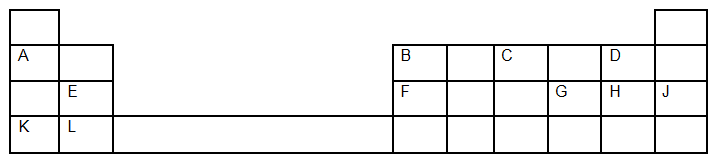
- The grid below show a section of the periodic table. The letters are not the actual chemical symbols"
- Which letter represents the
- Most reactive non metal. Explain your answer [2mks]
- Most reactive metal. Explain your answer [2mks]
- What name is given to the family into which element E belongs [1mk]
- Write the formulae of the compound formed between
- F and G [1mk]
- K and oxygen [1mk]
- How does the atomic radius of F and G compare? Explain [2mks]
- In terms of structure and bonding, explain why the oxide of F has a high melting point [2mks]
- State one possible use of
- Element J [1mk]
- Element E [1mk]
- Which letter represents the

MARKING SCHEME
-
-
- filtration √1
- its insoluble in Liquid L √1
- sand √1 and water √1
Both must be correct - Decantation√1
- Apparatus A- filter funnel √1
Apparatus B – conical flask √1 - used to deliver solution/ liquids carefully to apparatus with narrow necks. √1
Can be used in dissolving highly soluble gases in water to avoid sucking back effects√1
-
- Blue hydrated copper(ii) sulphate turns to white powder
Colourless liquid droplets on the cooler parts of the test tube √1 - water √1
- temporary chemical change √1 because change can be reversed√1
- Blue hydrated copper(ii) sulphate turns to white powder
-
-
- Zn(s) + CuSO4(aq) →ZnSO4(aq) + CU(s)√1
- To ensure all the copper(ii) sulphate solution was used up √1
- Blue colour of copper(ii) solution fades√1 because copper(ii) ions responsible for the colour are displaced from the solution√1
Brown solid is deposited √1 because copper(ii) ions are reduced to copper metal which is a brown solid. √1 -
-
-
-
-
- substance M- lead (ii) hydroxide/ pb(OH)2
- solid P- lead(ii) carbonate/ PbCO3
- gas R- carbon (iv) oxide √1
- Solution L- Nitric (v) acid √1
-
- Pb(OH)2(s) + 2OH-→ [Pb(OH)4]2-(aq) √1
- Pb2+(aq) + SO2-4(aq) → PbSO4(s) √1
-
- forms white ppt √1 insoluble in √1 excess ammonia
- H3O+ √1
- H2O(l)
-
-
- hydrogen gas
-
- Mg(s) + H2O(l)→ MgO(s) +H2(g) √1
- H2(g) + O2(g) →H2O(l) √1
- to drive out air that was initially in the glass tube so that it does oxidise the magnesium ribbon. √1
- clamp and stand √1
- mass of magnesium increases√1 because magnesium react with oxygen ,to form magnesium oxide powder. √1
- -less dense than air √1
-insoluble in water √1
-colourless and oudourless gas √1
Any two correct - -used in haber process to manufacture ammonia gas
-used in hardening of margarine(oil)
Any two correct - to generate steam that would react with magnesium ribbon. √1
-
- hydrogen gas √1
- H2(g) + Cl2(g) →2HCl(g) √1
- to increase the surface area over which the gas dissolves in water √1
-
- Electrolysis of brine
Cracking of long chain hydrocarbons
Any one correct - Electrolysis of fused sodium chloride or brine √1
- Electrolysis of brine
- to prevent explosion when chlorine reacts with hydrogen. √1
- removing mist from metal √1
Making of dyes and drugs √1 - open a bottle of concentrated ammonia√1and place it near hydrogen chloride gas, dense white fumes of NH4Cl are observed
-
-
- propane √1
- But-2-ene √1
- 5- bromo pent- 2- ene √1
-
- Bromo ethane √1
-
-
- used as a fuel. √1
- natural gas√1
Biogas √1
Crude oil √1
Any two correct
-
-
-
- D- its most electronegative √1
- K- its most electropositive √1
- Alkaline earth metals √1
-
- F2G3 √1
Accept Al2O3 - K2O √1
- F2G3 √1
- F is larger than G√1. G has higher nuclear charge hence electrons are pulled inwards more strongly. √1
- has giant ionic structure with strong ionic bonds. √1
-
- its used in light bulbs to provide an inert environment to prevent oxidation. √1
Used as an insulator in arch welding √1
Any correct - used in manufacture of magnesium hydroxide which is used as anti-acid medicine
Its low density alloy with aluminum is used in airplane construction
Any correct
- its used in light bulbs to provide an inert environment to prevent oxidation. √1
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY PAPER 2 - KCSE 2019 STAREHE PRE MOCK EXAMINATION (WITH MARKING SCHEME).
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students