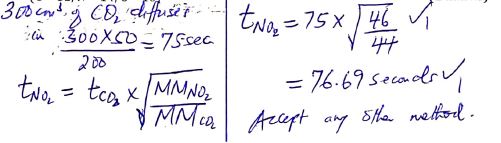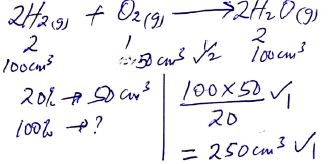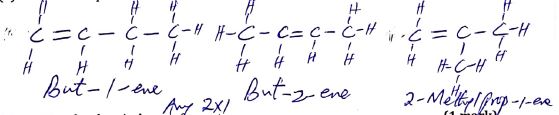INSTRUCTIONS TO CANDIDATES
- Write your name, admission number, date and school in the spaces provided.
- Answer all the questions in the spaces provided.
- All working must be clearly shown where necessary.
- Scientific calculators may be used.
FOR EXAMINERS’ USE ONLY
|
Questions |
Maximum Score |
Candidate’s Score |
| 1-26 | 80 |

QUESTIONS
- Ammonium nitrite was heated as shown below.
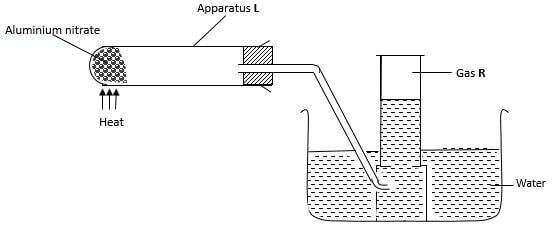
- Give a suitable material that can be used to make apparatus L, explain your answer. (2 marks)
- Write a chemical equation for formation of gas R. (1 mark)
- Dilute hydrochloric acid and sodium sulphite were reacted as shown below.
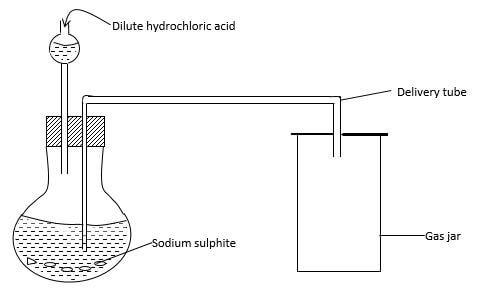
- Write a chemical equation for the reaction taking place in the flask. (1 mark)
- State one main laboratory rule that should be observed during the above experiment and give a reason for your choice. (2 marks)
- Give two reasons why no gas was collected in gas jar. (2 marks)
- In an experiment 3.36g of iron filling were added to excess copper (II) sulphate solution. Calculate the mass of copper that was deposited given that Iron (II) sulphate and copper were the products.
(Cu = 63.5, Fe = 56.0) (3 marks) - In a titration experiment 30cm3 of 2M sodium hydroxide required 30cm3 of sulphuric (VI) acid for complete neutralization. Determine the concentration of sulphuric (VI) acid in grams per litre. (3 marks)
- Study the diagram below and answer the questions that follow.
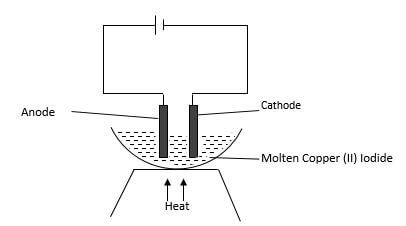
- State the observation made at the cathode. (1 mark)
- Write the equation taking place at the Anode. (1 mark)
- What material should be used to make the cathode? (1 mark)
-
- Define the term electrolysis. (1 mark)
- State two applications of electrolysis. (2 marks)
-
- Write the electron arrangement of element P which has atomic number 16. (1 mark)
- State the group and period of element P. (1 mark)
- Write the equation of element P when burnt in air. (1 mark)
- Study the diagram below and answer the question that follow.
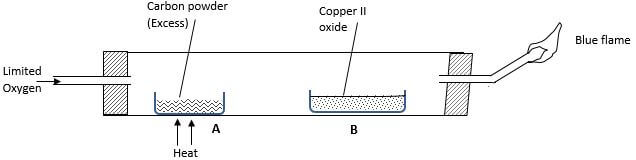
- State and explain observation made and point A. (2 marks)
- Explain the observation made out point B. (2 marks)
- Why was it necessary to burn the gas at the jet? (1 mark)
- Give two reasons why carbon (IV) oxide is used at a fire extinguisher. (2 marks)
- To determine the purity of limestone Form 3 students heated 12.5g of limestone in a crucible until they obtained a constant mass. If the volume of CO2 obtained was 2400. Calculate the purity of the limestone (C=12, O=16, Ca = 40, Mgv = 240dm3). (3 marks)
- Determine the relative atomic mass of Neon whose isotopic composition is as follows.
(3 marks)
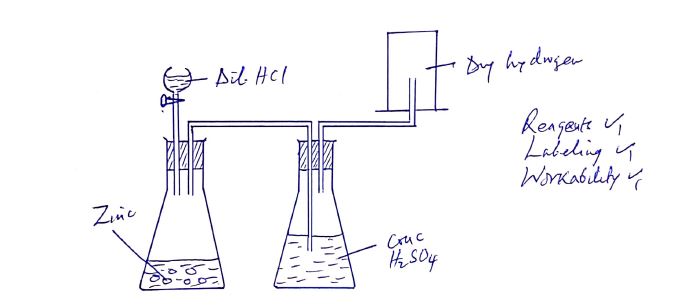
- Draw a well-labelled diagram to show how dry hydrogen can be prepared and collected in the laboratory. (3 marks)
- Give two characteristics of a temporary chemical change. State one example of such reaction. (2 marks)
-
- What is drug abuse? (1 mark)
- Name one commonly abused non-medicinal drug. (1 mark)
- A doctor prescribed drugs to a patient Amoxil 2x3. How should the patient take the drug? (1 mark)
- The figure below shows a flame obtained from a Bunsen burner.
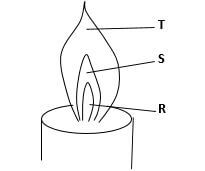
- Name the type of flame. (1 mark)
- A matchstick head placed at region R will not ignite. Explain. (1 mark)
- Name region S. (1 mark)
- The set-up below was used to separate a certain mixture.
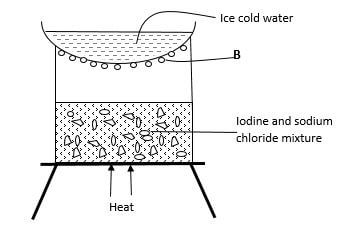
- Identify the method of separation shown. (1 mark)
- Identity substance B. (1 mark)
- Give any other substance when mixed with sodium chloride can be separated as above? (1 mark)
- Element A with atomic number 12 and B with atomic number 9.
- To which chemical family is; (2 marks)
A - ………………………………………………………………………
B - ……………………………………………………………………… - Write the equation for the reaction between A and B. (1 mark)
- To which chemical family is; (2 marks)
- When chlorine gas is passed over heated iron metal, 26.7g of the product is formed. Calculate the mass of iron which reacted. (Fe = 56, Cl = 35.5) (3 marks)
- Study the diagram below and answer the questions that follow.
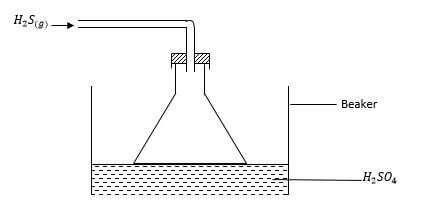
- Give the observation made in the beaker. (1 mark)
- Write an equation for the reaction that took place in the beaker. (1 mark)
- Give one reason why the gas is directed into the beaker using the inverted funnel as above? (1 mark)
-
- State Graham’s law of diffusion. (1 mark)
- It takes 50 seconds for 200cm3 of carbon (IV) oxide to diffuse through a plug.
How long will it take 300cm3 of nitrogen (IV) oxide to diffuse through the same plug under the same conditions of temperature and pressure.
(C = 12, N = 14, O = 16) (2 marks)
-
- State Gay Lussac’s law. (1 mark)
- Calculate the volume of air required to completely react with 100cm3 of hydrogen gas. (Assume that oxygen is 20% by volume of air). (3 marks)
- Name the following organic substances. (3 marks)
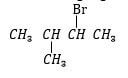 …………………………………………………………………
…………………………………………………………………- CH3CHCHCH2CH3 …………………………………………………………………
- CH3CH2COOCH2CH2CH3…………………………………………………………
-
- Define isomers. (1 mark)
- Draw the possible structural isomer of C4H8 (2 marks)
-
- Give the chemical name for rust. (1 mark)
- Name one condition which speeds up rusting. (1 mark)
- Many iron products are coated with a layer of zinc to protect it from rusting. State two ways in which zinc prevents rusting of iron. (1 mark)
- Study the diagram below for the preparation of oxygen in the laboratory.
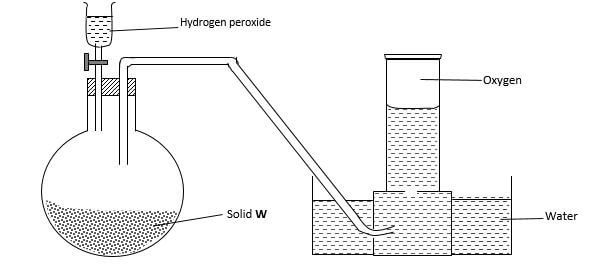
- Name solid W. …………………………………………………………………….. (1 mark)
- Write the equation for the reaction. (1 mark)
- What property of oxygen makes it to be collected as above? (1 mark)
- Starting with Barium Oxide describe how Barium chloride can be prepared in the Lab. (2 marks)

MARKING SCHEME
- Ammonium nitrite was heated as shown below.

- Give a suitable material that can be used to make apparatus L, explain your answer. (2 marks)
Glass; glass has a high melting point hence can withstand strong heating. glass is transparent for correct observation - Write a chemical equation for formation of gas R. (1 mark)
(heat)
NH4NO2(s) → N2 (g) + 2H2O(l)
- Give a suitable material that can be used to make apparatus L, explain your answer. (2 marks)
- Dilute hydrochloric acid and sodium sulphite were reacted as shown below.

- Write a chemical equation for the reaction taking place in the flask. (1 mark)
NaSo3 (s) + 2HCL(g) → 2NaCl(aq) + S02(g) + H2O(l) - State one main laboratory rule that should be observed during the above experiment and give a reason for your choice. (2 marks)
poisonous gases should be prepared in the fume chamber ; So2 produced is poisonous - Give two reasons why no gas was collected in gas jar. (2 marks)
-thistle funnel was dipped into the reaction mixture.-delivery tube entered the solution
- Write a chemical equation for the reaction taking place in the flask. (1 mark)
- In an experiment 3.36g of iron filling were added to excess copper (II) sulphate solution. Calculate the mass of copper that was deposited given that Iron (II) sulphate and copper were the products.
(Cu = 63.5, Fe = 56.0) (3 marks)
Fe(s) + Cu2+(aq) → Fe2+ (aq) + Cu(s)
moles of Fe = 3.36 = 0.06moles
56
moles of Cu formed = 0.06
mass of Cu formed = 6.3 × 0.06
3.81g - In a titration experiment 30cm3 of 2M sodium hydroxide required 30cm3 of sulphuric (VI) acid for complete neutralization. Determine the concentration of sulphuric (VI) acid in grams per litre. (3 marks)
2NaOH (aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O (l)
moles of NaOH = 30 × 2 = 0.06
1000
moles of H2SO4 = 0.06 = 0.03
2
molarity of H2SO4 = 0.03 × 1000 = 1M
30
conc. in g/l = 1 × 98 = 98g/l - Study the diagram below and answer the questions that follow.

- State the observation made at the cathode. (1 mark)
brown beads/brown solid - Write the equation taking place at the Anode. (1 mark)
2I(s) → I2(g) + 2e- - What material should be used to make the cathode? (1 mark)
graphite/platinum
- State the observation made at the cathode. (1 mark)
-
- Define the term electrolysis. (1 mark)
decomposition of molten/aqueous substance when electric current is passed through it - State two applications of electrolysis. (2 marks)
extraction of metals ef K, Na, Mg
putifcation of metals
electroplating
- Define the term electrolysis. (1 mark)
-
- Write the electron arrangement of element P which has atomic number 16. (1 mark)
2.8.6 - State the group and period of element P. (1 mark)
group VI
Period 3 - Write the equation of element P when burnt in air. (1 mark)
P(s) + O2(g) → PO2(g)
or
2P(s) + O2(g) → 2PO3(g)
- Write the electron arrangement of element P which has atomic number 16. (1 mark)
- Study the diagram below and answer the question that follow.

- State and explain observation made and point A. (2 marks)
red glow/yellow glow- rwaction is exothermic - Explain the observation made out point B. (2 marks)
black solid remains black - absence of heat prevented reduction of CuO by CO - Why was it necessary to burn the gas at the jet? (1 mark)
its poisonous
- State and explain observation made and point A. (2 marks)
- Give two reasons why carbon (IV) oxide is used at a fire extinguisher. (2 marks)
its denser than air
it does not support combustion - To determine the purity of limestone Form 3 students heated 12.5g of limestone in a crucible until they obtained a constant mass. If the volume of CO2 obtained was 2400. Calculate the purity of the limestone (C=12, O=16, Ca = 40, Mgv = 240dm3). (3 marks)
heat
CaCO3(s) → CaO(s) + Co2(g)
moles of Co2 = 2400 = 0.1
24000
moles of CaCo3 = 0.1
mass of Caco3 = 0.1 × 100
=10g
% purity = 10 × 100 = 80%
12.5 - Determine the relative atomic mass of Neon whose isotopic composition is as follows.
(3 marks)
RAM = (20 × 90.92) + (212 × 0.26) + (22 × 8.82)
100
=1818.4 + 5.46 + 194.04
100
=20.179 - Draw a well-labelled diagram to show how dry hydrogen can be prepared and collected in the laboratory. (3 marks)
- Give two characteristics of a temporary chemical change. State one example of such reaction. (2 marks)
reversible
new substance formed
change in mass
net heat change
Example: heating of CuSO4.5H2O
heating of CoCl2.6H2O -
- What is drug abuse? (1 mark)
use of drug for a purpose other than the one it is intended for/ overdose or underdose of a drug - Name one commonly abused non-medicinal drug. (1 mark)
cacaine/miraa/alcohol - A doctor prescribed drugs to a patient Amoxil 2x3. How should the patient take the drug? (1 mark)
2 tablets after every 8 hours
- What is drug abuse? (1 mark)
- The figure below shows a flame obtained from a Bunsen burner.

- Name the type of flame. (1 mark)
non-luminous flame - A matchstick head placed at region R will not ignite. Explain. (1 mark)
its a region of unburnt gases hence not very hot - Name region S. (1 mark)
green-blue zone
- Name the type of flame. (1 mark)
- The set-up below was used to separate a certain mixture.

- Identify the method of separation shown. (1 mark)
sublimation - Identity substance B. (1 mark)
iodine - Give any other substance when mixed with sodium chloride can be separated as above? (1 mark)
AlCl3/ solid Co2/FeCl3/ Benzoic acid
- Identify the method of separation shown. (1 mark)
- Element A with atomic number 12 and B with atomic number 9.
- To which chemical family is; (2 marks)
A - alkaline earth metals
B - Halogens - Write the equation for the reaction between A and B. (1 mark)
A(s) + B2(g) → AB2(s)
- To which chemical family is; (2 marks)
- When chlorine gas is passed over heated iron metal, 26.7g of the product is formed. Calculate the mass of iron which reacted. (Fe = 56, Cl = 35.5) (3 marks)
2Fe(s) + 3Cl2(g) → 2fecl3(s)
moles of FeCl3 = 26.7 = 0.1643
162.5
moles of Fe = 0.1643
mass of Fe = 0.1643 × 56
=9.20089 - Study the diagram below and answer the questions that follow.

- Give the observation made in the beaker. (1 mark)
a yellow solid deposit - Write an equation for the reaction that took place in the beaker. (1 mark)
H2SO4(aq) + 3H2S(g) → 4S(s) + 4H2O(l) - Give one reason why the gas is directed into the beaker using the inverted funnel as above? (1 mark)
to avoid sucking back the acid
to increase S.A for the reaction
- Give the observation made in the beaker. (1 mark)
-
- State Graham’s law of diffusion. (1 mark)
under constant temp and pressure, the rate of diffusion of a gas is inversely proportional to square root - It takes 50 seconds for 200cm3 of carbon (IV) oxide to diffuse through a plug.
How long will it take 300cm3 of nitrogen (IV) oxide to diffuse through the same plug under the same conditions of temperature and pressure.
(C = 12, N = 14, O = 16) (2 marks)
- State Graham’s law of diffusion. (1 mark)
-
- State Gay Lussac’s law. (1 mark)
when gases react they do so in volumes that bear a simple ration to one another and to the volume of the products if gaseous temp and pressure remain constant - Calculate the volume of air required to completely react with 100cm3 of hydrogen gas. (Assume that oxygen is 20% by volume of air). (3 marks)
- State Gay Lussac’s law. (1 mark)
- Name the following organic substances. (3 marks)
 2-bromo-3-methylbutane rej; 3-bromo-2-methylbutane
2-bromo-3-methylbutane rej; 3-bromo-2-methylbutane- CH3CHCHCH2CH3 pent-2-ene
- CH3CH2COOCH2CH2CH3 propylpropanoate
-
- Define isomers. (1 mark)
compounds with same molecular formula but different structural formula - Draw the possible structural isomer of C4H8 (2 marks)
- Define isomers. (1 mark)
-
- Give the chemical name for rust. (1 mark)
hydrate Iron(III) oxide - Name one condition which speeds up rusting. (1 mark)
acidic condition/ high temp/ salt condition - Many iron products are coated with a layer of zinc to protect it from rusting. State two ways in which zinc prevents rusting of iron. (1 mark)
cathodic protection
galvanisation
- Give the chemical name for rust. (1 mark)
- Study the diagram below for the preparation of oxygen in the laboratory.

- Name solid W. Manganese(iv)oxide; rej; formula (1 mark)
- Write the equation for the reaction. (1 mark)
MaO2
2H2O2(l) → 2H2O(l) + O2(g) - What property of oxygen makes it to be collected as above? (1 mark)
slightly soluble in water/does not react with water
- Starting with Barium Oxide describe how Barium chloride can be prepared in the Lab. (2 marks)
add excess BaO into HCL(Aq) and stir
filter to remove excess BaO as residue
heat the filtrate to saturation
allow it to cool
pour out the molten liquor
dry the crystals between filter papers
Download Chemistry Paper 1 Pre Mock Questions and Answers - Mokasa I Joint Examination July 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students