CHEMISTRY
PAPER 1 :THEORY
INSTRUCTIONS TO CANDIDATES
- Write your name and index number in the spaces provided above
- Sign and write the date of examination in the spaces provided.
- Answer all the questions in the spaces provided.
- Mathematical table and silent electronic calculators may be used.
- All working must be clearly shown where necessary.
- Candidates should answer the questions in English
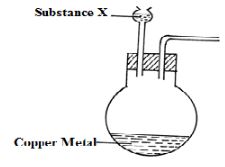
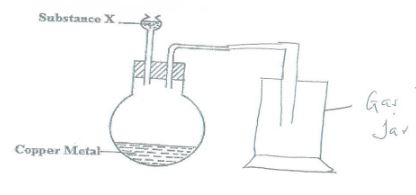
- The set shown below is used to prepare nitrogen (IV) oxide.
- Complete the diagram to show the collection of the gas. (1 mark)
- Identify substance X. (1 mark)
- Write the equation for the reaction that occurs in the conical flask. (1 mark)
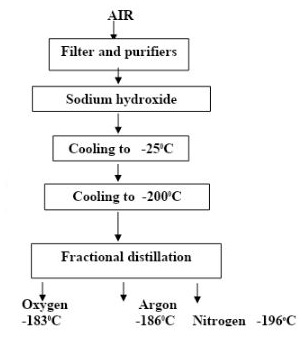
- The flow chart below shows stages involved in obtaining nitrogen gas from air
State the use of the,- Filters (1mark)
- Sodium hydroxide solution (1mark)
- Cooling to -200ºCelsius. (1mark)
- The electron arrangement of ions X3+ and Y2- are 2. 8 and 2. 8. 8 respectively.
- To which groups do X and Y belong to? (1 mark)
- State the atomic numbers of X and Y. (1 mark)
- Write a formula of compound formed when Y and X reacts. (1 mark)
- In the extraction of iron in the blast furnace, state the uses of the following in the furnace.
- Molten slag. (1 mark)
- Waste gases leaving the furnace. (1 mark)
-
- Water from Nairobi dam is suspected to be hard water with a little presence of chloride ions from the industrial effluents. Give the formulae of two ions that cause hardness of water and describe a chemical test to detect the presence of chloride ions . (3 mark)
- Given that the structure of soap is C17H35COONa, use ionic equations to show the reaction that occurs when the above ions react with soap. (2 marks)
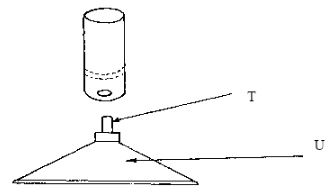
- The diagram below shows some parts of a Bunsen burner
Explain how the parts labelled T and U are suited to their functions (2marks) - Ethyne reacts with hydrogen as shown below
Use the bond energies below to calculate the enthalpy changes for the above reaction (3marks)
BOND ENERGY(kJ/Mole) H-H 435 C-H 413 C≡C 835 C=C 611 -
- What is meant by the term isomerism? (1mark)
- Draw structural formulae of two positional isomers of pentene. (2marks)
- 18.7cm3 of a dibasic acid (H2A) required 25cm3 of 0.1M Sodium hydroxide for complete neutralization.
- How many moles of sodium hydroxide are contained in 25cm3? (1mark)
- Calculate the molarity of the acid. (2marks)
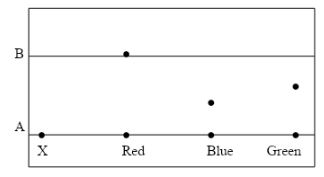
- The figure below shows a paper chromatography for a pure substance X
- What do the letters A and B represent? (1 mark)
- Given that X contains blue and green dyes, show the chromatography of X on the diagram. (1 mark)
- Give the reason why the spot for red moves further than that of blue and green. (1 mark)
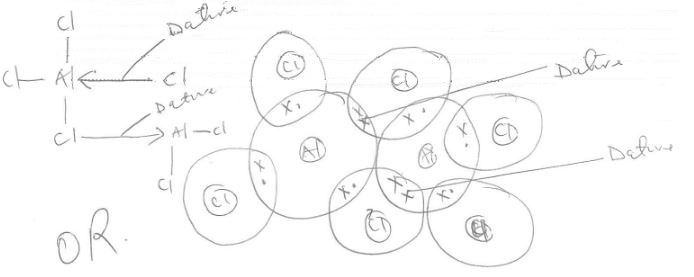
- Draw the dimer structure of aluminium chloride and name the bonds. (2 marks)
(Atomic number Al = 13, Cl =17) - One of the applications of electrolysis is electroplating of iron.
- Give two reasons why iron is electroplated. (1 mark)
- An iron spoon was to be electroplated with silver. Draw the labelled set-up that can be used. (2 marks)
-
- Distinguish between isotopes and allotropes. (2marks)
- Other than sulphur, name two elements that are allotropic. (1mark)
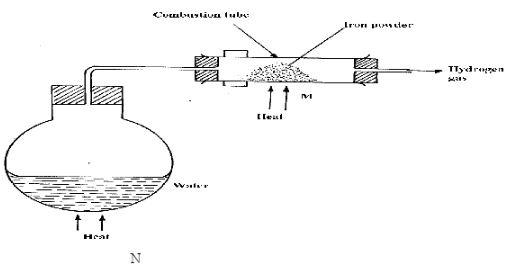
- Study the diagram below and answer the questions that follow.
- Between N and M which part should be heated first? Explain (2marks)
- Write the equation for the reaction occurring in the combustion tube. (1mark)
- Describe how you would prepare crystals of sodium nitrate starting with 200 cm3 of 2M sodium hydroxide. (3 marks)
- Two reagents that can be used to prepare chlorine gas are potassium manganate (VII) and hydrochloric acid.
- What is the role of potassium manganate (VII) in this reaction? (1 mark)
- Give the formula of another reagent that can be used instead of potassium manganate (VII). (1 mark)
- Using an equation, illustrate how chlorine bleach coloured substances. (1 mark)
- A certain hydrocarbon on complete combustion gave 9.9g of carbon (IV) oxide and 4.86g of water. Calculate the empirical formula of the hydrocarbon (3marks)
- Magnesium hydroxide is used as a medication to relieve stomach acidity.
- Write the equation for the reaction that occurs in the stomach when one takes in the medicine. (1 mark)
- What type of reaction takes place in the stomach after taking the medicine? (1 mark)
- Sodium hydroxide cannot be used for the same purpose. Explain. (1 mark)
- The table below shows atomic numbers of elements represented by the letter R to Y. The letters are not the actual chemical symbols of the elements.
Elements R S T U V W Z Y Atomic number 3 7 8 9 10 11 12 13 - Two elements that belong to the same period of the periodic table. (1 mark)
- Two elements in the same group (1 mark)
- State one use of the element V. (1 mark)
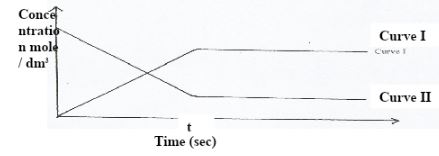
- The curve below represent the changes in the concentrations of substance E and F with time in the reaction
- Which curve represents the changes in the concentration of substance F? Give a reason. (2 marks)
- Give a reason for the shapes of the curves after time (t) seconds. (1 mark)
-
- Define the term solubility. (1 mark)
- The following were the results obtained in an experiment to determine solubility of potassium nitrate at room temperature.
Mass of evaporating dish = 14.32 g
Mass of evaporating dish + saturated solution = 35.70 g
Mass of evaporating dish + salt (residue) = 18.60 g
Calculate the solubility of potassium nitrate from the above results. (2 marks)
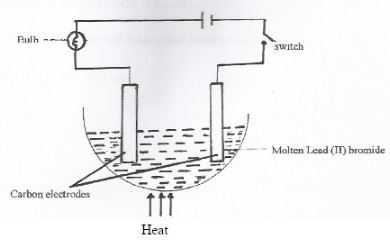
- Study the set-up below and answer the questions that follow;
- State and explain two observations made when the circuit is completed. (2marks)
- What precaution should be taken when performing this experiment? Give a reason (l mark)
- Dry carbon (II) oxide gas was passed over heated lead (II) oxide.
- Write the equation for the reaction. (1 mark)
- Give the industrial application of the above reaction. (1 mark)
- Name another gas that can be used in the above reaction. (1 mark)
-
- Consider the reaction shown below.
2H2S(g) + SO2(g) → 3S(s) + 2 H2O(l)
Using oxidation numbers of sulphur in H2S and SO2, identify the reducing agent. Explain (2marks) - What name is given to the process used to control pollution caused by sulphur (IV) oxide in a sulphuric (VI) acid plant?( 1mark)
- Consider the reaction shown below.
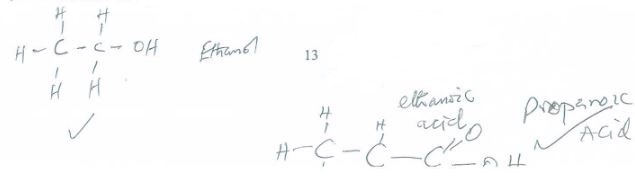
- The following is a formula of an organic compound:CH3CH2COOCH2CH3
- Draw and name the organic acid and alkanol used in making the compound. (2 marks)
- Name the compound and the gas formed when the alkanol in (a) above is reacted with potassium. (1 mark)
-
- State Graham’s law of diffusion. (1 mark)
- A certain volume of gas T takes 180 seconds to diffuse through a porous plug. An equal volume of gas Q takes 240 seconds to diffuse through the same plug. Calculate the molar mass of Q. (2marks)
(Relative molecular mass of gas T =l8)
- Sulphur IV oxide gas dissolves in water to form sulphuric IV acid (H2SO3),
- Write the electronic arrangement of Suphur in the compound H2SO3 (2mks)
MARKING SCHEME
-
-
- X
- Concentrated Nitric V Acid - Reaction:
Cu(s) + 4HNO3(l) → Cu(NO3)2(aq) + 2H2O(l) + 2NO2(g)
-
-
- Filters - Remove dust particles/ impurities
- Sodium hydroxide solution - Remove Carbon (IV) Oxide
- Cooling to -200º celsius - Condense gases into liquid
-
- X - III
Y - VI - X - 13
Y - 16 - X2Y3
- X - III
-
-
- Mg2+ or Ca2+
Test - Add Silver NItrate ( AgNO3) to the sample and a white precipitate is formed. - 2C17H15COO+ + Ca2+ → (C17H15COO)2Ca8
or
2C17H15COO+ + Mg2+ → (C17H15COO)2Mg15
- Mg2+ or Ca2+
- T - Jet - Divert the gas to enter the chiney, forcing air to flow out
U - Stand - Wide base and heavy to support burner - (2(H-C)+(C≡C) + (H-H) - 2(-C-H) + (C=C)
(2(473) + 835 + 435) - 2(413) + 611
826 + 835 + 435
2096 - 1222
= -874KJ/No -
- Compound with the same chemical formula but different in their structural formula
-
- If 0.1 moles NaOH - 1000
25 x 0.1 = 0.0025 moles
1000 - 2NaOH: 1H2A → Na2A + H2O
0.0025 moles of H2A - 1/2 x 0.0025
=0.00125
if 0.00125 moles - 18.7cm3
? - 1000cm3
1000 x 0.00125 = 0.066845
18.7
- If 0.1 moles NaOH - 1000
-
-
- Base line
- Solvent front
-
- Red is the most soluble dye
- Red is the least absorbent dye
-
-
-
-
- Isotope - as atoms of the same element will the same atomic number but different mass number
Allotropes - different forms of an element but the same physical state. - Carbon, phosphorus
- Isotope - as atoms of the same element will the same atomic number but different mass number
-
- N - water is heated to produce steam to expel thin air in combustion heating iron to avoid the formation of reaction of iron with oxygen.
- 2Fe(s) + 3H2O(l) → Fe2O3(s) + 3H2(g)
- prepare 200cm3 of 2m nitric(V) acid in a beaker. Add all the 200cm3 of 2m sodium hydroxide. To the result of solutionheat boil and evaporate the obtain the crystals.
-
- oxidising agent
- Lead (iv) oxide
Manganese (iv) oxide - Cl2(g) + H2O(l) → HOCl(aq) + HCl(aq)
HOCl(aq) + dye coloured flower → HCl + [dye + O](white)
- 12/44 x 9.9 = 2.699997
2/18 x 4.86 = 0.539999
C H 2.7g
120.54
12.7/12 0.54 0.225/0.225 0.54/0.225 1 2.4 1 x 5 2.4 x 5 5 12 C5H12 -
- Mg(OH)2(aq) + 2HCl(aq) → MgCl2(aq) + H2O(l)
- Neutralisation
- Sodium hydroxide is a drug base. It affects the stomach linings/walls
-
- RSTUV or
WZY - R and W
- Street/ advertisement lights
Provide inert environemnt for bulbs
- RSTUV or
-
- Curve I - concentration increases with time until equilibrium
- The rate of forward/ backward reaction is attained (Dynamic equilibrium is attained)
-
- The maximum amount of a solid that can saturate 100g of water at a given temperature
- Mass of saturated solution = 35.70 - 14.32 → 21.38g
Mass of dry salt = 18.60 - 14.32 → 4.28
Mass of water → 21.38 - 4.28
= 17.1g
If 17.1g H2O - 4.28 Salt
100g H2O - ?
100 x 4.28 = 25.029g/100gH2O
17.1
-
-
- Bulb lights
- Grey beads of lead metal are deposited at the cathode
- Red-brown vapour of bromine are formed at the anode
-
- Use a gas mask/ do the experiment in the fume chamber to avoid inhaling bromine gas. It is corrosive to humn tissue, irritates eyes.
-
-
- 2PbO(s) + 2CO(g) → 2Pb (s) + 2CO2(g)
- In extraction of lead metal/metals
- Hydrogen
ammonia
-
- Hydrogen sulphide.
- Scrubbing
-
-
- Potassium ethoxide
Hydrogen gas
-
-
- Rate of difussion of a gas is inversely proportional to the square root of the density provided temperature and pressure remain constant.
- RA/RB = √MB/MA = √DB/DA = Tm A/TmB
T/Q = √MT/MQ = 180/240 = √18/Q
- H2SO3 =0
(+2) + S +(-6)=0
S=(+6 -2)
S=+4
Download Chemistry Paper 1 Questions and Answers - Kapsabet Pre Mock Exams 2021/2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students