QUESTIONS
- Bronze is an alloy of Tin and Copper. Give one use of Bronze (1mk)
- The following setup was used to investigate some properties of two gases G and H
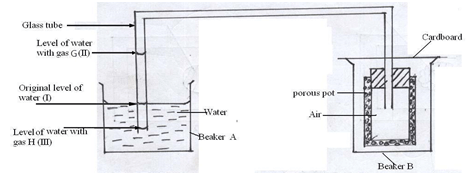
When beaker B was filled with gas G the level of water in the glass tube rose to point II. When the experiment was repeated using gas H, the level of water dropped to point III. Explain these observations (3mks) - State the oxidation number of Manganese in
- MnO2(1mk)
- MnO-4(1mk)
- Chlorine can be prepared by using the following three reagents; Solid Sodium Chloride, Concentrated Sulphuric (VI) acid and Potassium Manganate (VII).
What is the role of each of the following in the reaction?- Concentrated Sulphuric (IV) acid (1mk)
- Potassium Manganate (VII) (1mk)
- Which other reagent can be used instead of solid Sodium Chloride? (1mk)
- The set up below was used to electroplate a metallic door handle. Study it and answer the questions that follows.
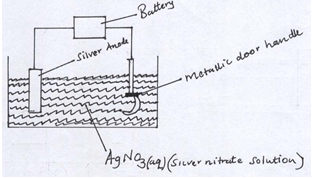
- Write an ionic equation for the reaction that occurred at the cathode. (1mk)
- State and explain what happens to the anode. (2mks)
- Patience Masafu of form II at Mumias Muslims Girls high school set up the following experiment with the help of the two laboratory assistants. Metal rods S, T, U and V were cleaned with sand paper and placed in a beaker containing water. A second set was put in a container of steam and a third set was placed in a beaker containing a dilute acid. Bubbles of gas and reactions were observed around some of the rods as shown in the diagrams below.
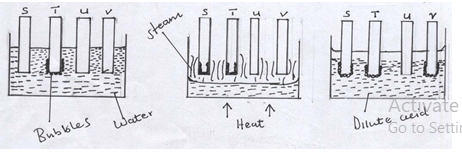
- It was very necessary to clean the rods with sand paper before dipping them. Explain(2mks)
- Arrange the four metals in order of their reactivity starting with the most reactive. (2mks)
- Describe how a solid sample of Lead (II) Chloride can be prepared using the following reagents:
Dilute Nitric Acid, Dilute hydrochloric acid and Lead Carbonate.(3mks) - Using Bronsted and Lowry theory, define the terms:
- A base(1mk)
- An acid(1mk)
- How is this different from Arrhenius definition? (2mks)
- Study the energy level diagram below and answer the questions that follow
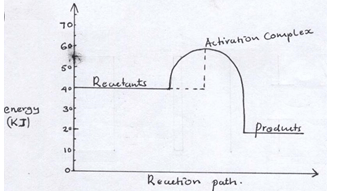
- State and explain whether the reaction represented in the diagram is endothermic or exothermic.(2mks)
- From the diagram, determine
- The activation energy.(1mk)
- Enthalpy of reaction(1mk)
- Explain why the reaction between 1.0g of Calcium Carbonate and 1M Hydrochloric acid is faster than the reaction between 1.0g Calcium Carbonate and 1M at Butanoic acid(3mks)
- Hopson, a form four student at Mwitoti high School wanted to determine the solubility of Potassium Nitrate. He obtained the following results as shown below.
Mass of evaporating dish 15.13g
Mass of evaporating dish and solution 36.51g
Mass of evaporating dish and salt 19.41g
Use the information above to calculate the solubility of Potassium Nitrate (3mks) - State three factors that should be considered when choosing fuel for cooking. (3mks)
- A sealed glass tube containing air at s.t.p was immersed in water at 80ºc. Assuming there was no increase in the volume of the glass tube due to expansion of the glass, calculate the pressure of the air inside the tube.(3mks)
(Standard pressure = 760mmHg, Standard Temperature = 273k) - During electrolysis of aqueous Zinc Sulphate solution, a current of 0.64 A was passed through the electrolyte for 18 minutes. Calculate the volume of gas produced at the anode. (1 Faraday = 96500 coulombs, Molar gas volume is 24000cm3 at room temperature) (3mks)
- The elements shown in the table belong to a certain metallic group in the periodic table. Study the information and answer the questions that follow.
Define the termElement Atomic size (nm) S 0.160 T 0.180 V 0.193 - Ionization energy.(1mk)
- Which element is likely to have most ionization energy? Explain(2mks)
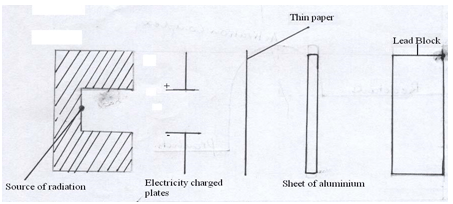
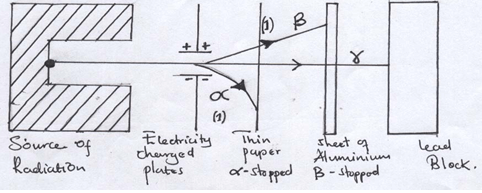
- Complete the diagram below to show how Alpha and Beta particles from radioactive source can be distinguished from each other. Label your diagram clearly. (2mks)
The following are nuclear equations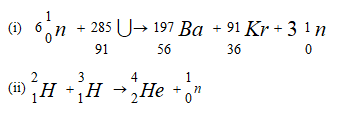
Identify the nuclear fission reaction. (1mk) - Painting, Oiling, galvanizing and or tin plating are methods of rust prevention.
- Explain the similarity of these methods in the ways they prevent rusting.(1mk)
- Explain why galvanized iron objects are better protected even when scratched. (1mk)
- Write down the chemical formula for rust(1mk)
-
- State and explain Boyle’s law on the behaviour of gases. (2mks)
- State two conditions under which real or natural gases are likely to behave like ideal or perfect gases.(1mk)
-
- Name the following compounds.
- CH3CH2CH2C-OH (1mk)
- CH3COOCH2CH2CH3 (1mk)
- Complete the following equation.
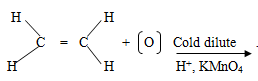
- Name the following compounds.
- Study the diagram below and answer the questions that follow. The diagram shows the method used to separate components of mixture Q
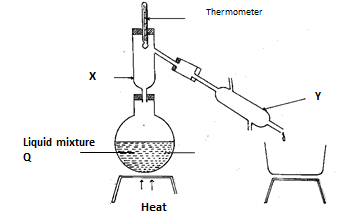
- Name X and Y (1mk)
- What is the purpose of apparatus X? (½ mk)
- Show the direction of flow of cold water used for cooling the vapour formed. (½ mk)
- What name is given to the above method of separating mixtures? (1mk)
- Explain the following observations.
- Alkaline earth metals are generally less reactive than alkali metals. (1mk)
- The order of reactivity increases down group I, but decreases down group VII. (2mks)
- Describe briefly a simple test which would be used to distinguish between the following ions in the solution. Give the result of the test in each case.
- Zn2+(aq) and Fe2+(aq) (1½mks)
- CO32-(aq) and SO32-(aq) (1½mks)
- The flow chart below shows laboratory preparation of chlorine gas. Study it and answer the questions that follow.
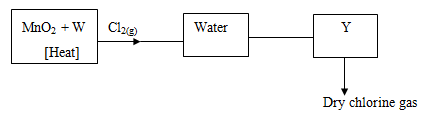
- Name substances. (1mk)
- Explain whether heating would be necessary if Potassium Manganate (VII) was used instead of Manganese (IV) Oxide (MnO2) (1mk)
- What is the function of water in the above set up? (1mk)
-
- What condition is necessary for chemical equilibrium to be established?(1mk)
- The production of ammonia gas involves a reversible reaction as shown
N2(g) + 3H2(g) ⇌ 2NH3(g) ΔH = - ve
Suggest two conditions that are likely to shift the equilibrium from right to left. (2mks)
- An unknown mass of anhydrous potassium carbonate was dissolved in water and the solution made upto 200cm3. 25cm3 of this solution neutralized 18.0cm3 of 0.22M nitric (v) acid. Calculate the unknown mass of potassium carbonate (K=39, C=12, O = 16) (3mks)
- When magnesium ribbon is burnt in air and the product dissolved in water, a colourless solution is formed and a colourless gas is evolved.
- Name the compound responsible for the production of the colourless gas. (1mk)
- Write down a balanced chemical equation for the reaction producing the colourless gas. (1mk)
- The following are some half-cell electrode potentials with respect to copper.
E/V
K+(aq) + e- → K(s) -2.99
Na+(aq) + e- → Na(s) -2.75
Ca2+(aq) + 2e- → Ca(s) - 2.86
Cu2+(aq) + 2e- → Cu(s) 0.00
Hg2+¬(aq) + 2e- → Hg(l) +0.87
Ag+(ag) + e → Ag(s) + 0.79- Explain why electrode potential of copper is zero. (1mk)
- Identify the weakest oxidizing agent and weakest reducing agent. (1mk)
- Weakest oxidizing
- Weakest reducing
- Work out the e.m.f of a cell represented by
Na(s) I Na+(aq) II Ag+(aq) I Ag(s) (1mk)

MARKING SCHEME
- For decoration or ornamental (medals) (1) (1mk)
- With G, there is a reduction in pressure in the pot ( ½ ); because air moves out faster than G enters ( ½ ); G is denser// air is lighter than G ( ½ ) with H, there is an increase in pressure inside the pot ( ½ ); because H enters the pot faster than air comes out ( ½ ); it is less dense than air//air is denser than H ( ½ ) (3mks)
-
- +4 (1)
- +7 (1) (2mks)
-
- drying agent (1)
- Oxidizing agent (1)
- Hydrochloric acid (1) (3mks)
-
- Ag+(aq) + e- Ag(s) (1)
- The anode dissolves (1); this is because it is active (1) (3mks)
-
- To remove the layer of oxide (1) on their surfaces which could inhibit the reaction (1)
- T, S, V, U (1) ( any correct two only (1)) (4mks)
- Dissolve ( ½ Lead carbonate in dilute Nitric acid ( ½ ) React the mixture with dilute Hydrochloric acid (1) Filter ( ½ ); to get Lead (ii) Chloride ( ½ )
-
- A base is a proton acceptor (1)
- An acid is a proton donor (1) (4mks)
- Difference in Arrhenius definition (2)
-
- Exothermic (1); the products are at a lower energy level than the reactants. Thus energy was lost to the environment. (1)
- Activation energy 60 – 40 = 20 KJ(1)
Enthalpy of the reaction 20 – 40 = -20KJ ( ½ )
ΔH = -20KJ ( ½ )
- Hydrochloric acid is a stronger acid than Butanoic acid (1); thus Hydrochloric acid undergoes complete dissociation unlike Butanoic acid which dissociates only slightly. (2) (3mks)
- Residue mass = 19.41 – 15.13 = 4.28g
Mass of solvent = 36.51 – 19.41 = 17.09g
17.09g are saturated by 4.28g solute
100g are saturated by 4.28 x 100
17.09
=25.04g/100g water - Heating value (amount of heat energy produced) (1)
Type of flame produced (case of combustion) (1)
Portability convenience (1) etc
P1 = P2
T1 T2
P2 = P1T2
T1 - P2 = 760 x 353 = 982.71
273
= 782.7mmHg
(3mks) - Q = 0.6 x 18 x 60
= 691.2 Coulombs (1)
1mole of gas (O2 requires 4 faraday) i.e
4OH-(aq) → 4e-(g) + 2H2O + O2(g)
691.2 x 1 = 0.00179 moles
4 x 96500
Volume of O2 = 24000 x 0.00179
= 42.98cm3 -
- Ionisation energy is the energy released or absorbed when an atom gains or loses an electron (1)
- S (1); it has the smallest atomic radius and so the outermost electrons are strongly attracted to the close nucleus. More energy is therefore required to get the electron off the energy level (1)
 Is fission
Is fission-
- They prevent air from getting in touch with iron.
- The protective layer will still overlay the iron even if it is scratched, and will still act as a sacrificial metal as long as it is connected with iron.
- Fe2O3.5H2O
-
- The volume a fixed mass of gas is inversely proportional to its pressure at constant temperature
Particles of a gas are widely spaced hence can be compressed - Low pressure
High temperature
- The volume a fixed mass of gas is inversely proportional to its pressure at constant temperature
-
- Butanoic acid
Propylethanoate
- Butanoic acid
-
- X - Fractionating column
Y - Liebig condenser - to condense back the component of higher boiling point
- shown on the diagram
- fractional distillation 1 mk
- X - Fractionating column
-
- They have a higher nuclear charge hence electrons are firmly held/more energy needed to lose the valence electrons than in group
- Down group 1 atomic size increases while nuclear attraction reduces hence ease of electron loss while down group 7 increased atomic size reduces the attraction for the incoming electrons / tendency to repel incoming electron increases down the group? 1 mk
-
- Add NH3 (aq) / NaoH (aq)
Zn2+ - white ppte soluble in excess
Fe2+ - dirty green ppte insoluble in excess - Add acidified K2Cr2O7 -- CO32- has no effect
SO23 - changes colour of K2Cr2O7 from orange to green
CO32- - no effect
SO32- - KMnO4 is decolourized
- Add NH3 (aq) / NaoH (aq)
-
- W - Conc. hydrochloric acid
Y – Conc sulphuric acid - Not necessary
KMnO4 is a stronger
oxidizing agent than MnO2 - Remove traces of HCL fumes
- W - Conc. hydrochloric acid
-
- Reaction carried out in a closed system or vessel
- Increase in temperature
Reduction in pressure
- Moles of acid = 18 x 0.22
1000
Moles of carbon ½ x 18 x 0.22
1000
concentration of carbon = 18 x 0.22 x 1000
2000 25
= 0.0792
mass in 200 cm3= molar mass x vol in litre x concentration
138 x 200 x 0.0792
1000 -
- It turns red litmus paper blue and has no effect on blue litmus papers
- Magnesium nitride
- Mg3N2 (s) + 6H2O(l) → 3Mg(OH)2(s) + 2NH2(gs)
-
- Two half of the same element have 0.00 potential or copper is the reference electrode
- Weakest oxidizing is K; weakest reducing is Ag+.
- 0.79 - (- 2. 75) =+ 3.54V
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Mumias West Pre Mocks 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

