INSTRUCTION TO CANDIDATES
- Write your name and Adm number in the spaces provided above
- Sign and write the date of the examination in the spaces provided
- Answer all the questions in the spaces provided
- All working must be clearly shown where necessary.
- Candidates should check to ascertain that each page s printed as indicated and that no question is/are missing.
FOR EXAMINAER’S USE ONLY
|
Question |
Maximum score |
Candidate’s score |
|
1-28 |
80 |
|

QUESTIONS
-
- State Graham’s law of diffusion. (1mk)
- 50cm³ of nitrogen (ii) oxide was allowed to diffuse through a porous membrane in 20 seconds. Calculate the time taken by equal volume of carbon (ii) oxide to diffuse through the same membrane. (C=12, N=14, O=16). (2mks)
- State two functions of a fume chamber in a laboratory. (2mks)
- The diagram below shows a structure of water molecule.
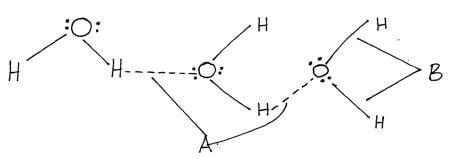
Name the bonds labelled. (2mks)- A
- B
- Two samples of water were put in separate beakers. They were boiled for sometime and allowed to cool. Equal volumes of soap were added to each sample and stirred. Water in beaker C readily formed lather with soap while water in beaker D required more soap to lather.
- Write the formula of one salt likely to be in water in beaker. (2mks)
- C
- D
- Name one method that can be used to soften water in beaker D. (1mk)
- Write the formula of one salt likely to be in water in beaker. (2mks)
- Describe how you would prepare lead (ii) sulphate given the following reagents: dilute nitric (v) acid, distilled water, sodium sulphate solid and lead metal. (3mks)
- During manufacture of sulphuric (vi) acid, sulphur (iv) oxide is oxidised to sulphur (vi) oxide in the presence of vanadium oxide catalyst as shown below:
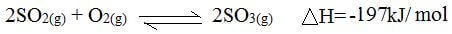
The reaction is carried out at a pressure of 3 atmospheres and a temperature of 450ºC. State and explain the effect on the yield of sulphur (vi) oxide if the reaction is:- Carried out at 3 atmospheres and 600ºC. (2mks)
- In absence of a catalyst. (2mks)
-
- Hydrogen gas was passed over 4.64g of an oxide of iron in a combustion tube until there was no further change. The mass of the final substance was found to be 3.36g. Determine the empirical formula of the oxide. (Fe=56, O= 16). (3mks)
- State the property of hydrogen demonstrated in the experiment above. (1mk)
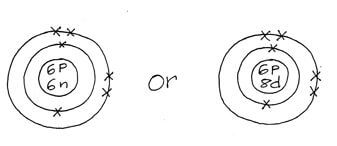
- Atoms of element X exist as 14 6X and 12 6 X.
- What name is given to the two types of atoms? (1mk)
- Use dot (.) and (x) diagram to represent electrons draw the atomic structure of x. (2mks)
- Hydrogen sulphide gas was passed through a solution of iron (ii) chloride.
- State two observations made. (2mks)
- Write an equation for the reaction taking place in (i) above. (1mk)
- Two clean iron nails of the same size were connected with wire to magnesium and silver stripes as shown.

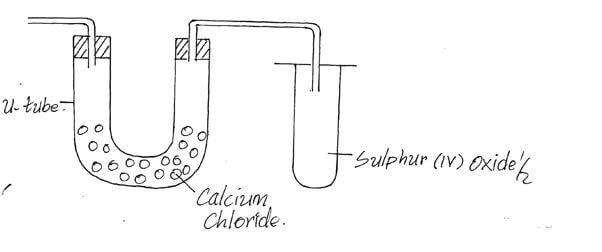
State and explain the observation made on nail x and y if they were left in the open for 2 weeks. (2mks) - The diagram below shows an incomplete setup used to prepare sulphur (iv) oxide in the laboratory.
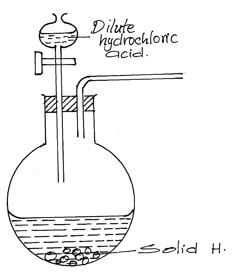
- Identify solid H. (1mk)
- Complete the set up above to show how dry sulphur (iv) oxide may be collected. (2mks)
- Some average bond energies are given below.
Determine whether the reaction below is exothermic or endothermic. (3mks)Bond
Energy in kJ/MOL
C-C
348
C-H
414
Cl- Cl
243
H-Cl
340
C – Cl
432
C2 H6(g) + Cl2(g) → C2H5Cl(g) + HCl(g) - Study the scheme below and answer questions that follow
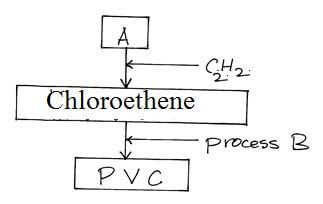
- Identify reagent A. (1mk)
- Name process B (1mk)
- What does PVC stand for? (1mk)
- Ethanedioic acid (H2C2O4) is used instead of methanoic acid (HCOOH) to prepare carbon (ii) oxide in the laboratory. It gives equal volume of carbon (ii) oxide and carbon (iv) oxide.
- Write an equation for the dehydration of ethanedioic acid. (1mk)
- Explain how pure carbon (ii) oxide can be obtained from the mixture. (2mks)
- The diagram below represents a set-up of apparatus used to investigate the effect of an electric current on lead (ii) bromide.
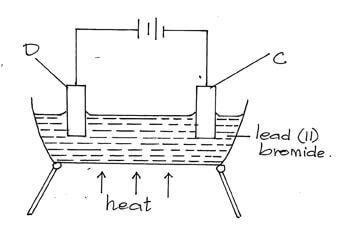
- Describe the observation made at electrode C. (2mks)√
- State two applications of electrolysis. (1mk)
- The set up below was used to investigate a property of ammonia gas.
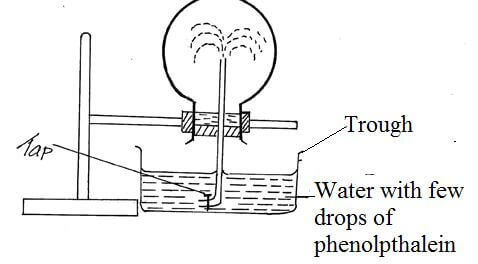
- What property of ammonia gas is being investigated? (1mk)
- The experiment above is commonly known as ‘the fountain experiment’; explain. (2mks)
- Identify another gas that may be used instead at ammonia gas. (1mk)
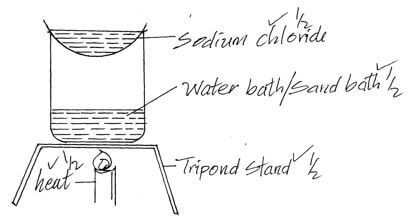
- Draw a well labelled diagram to show how crystals of sodium chloride can be obtained from sodium chloride solution. (3mks)
-
- Define the term solubility. (1mk)
- 40g of a saturated solution yields 15g of salt when evaporated to dryness. Calculate the solubility of the salt. (2mks)
- Increased levels of carbon (ii) oxide leads to global warming. Give two reasons why the amount of carbon (iv) oxide in the atmosphere is increasing gradually. (2mks)
- Explain the observation made when a blue litmus paper is dipped in methylbenzene in which hydrogen chloride gas is bubbled through. (2mks)
- The reaction between hydrogen gas and oxygen releases energy. A student drew the reaction profile for the reaction between hydrogen gas and oxygen gas.
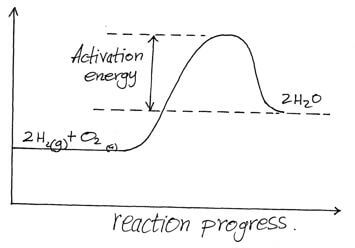
State two errors made when drawing the reaction profile. (2mks) - Both water (18) and hydrogen sulphide (34) are molecular substances. However water has a higher boiling point than hydrogen sulphide. Explain. (2mks)
- The grid below represents part of a periodic table. Study it and answer the questions that follow.
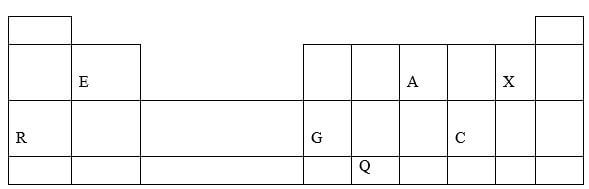
- How do the atomic radii of R and G compare. (1mk)
- How do the pH of the oxides of A and E compare. (1mk)
- On the grid, indicate with a tick (√) the position of K which is found on the third period and forms K3- ions. (1mk)
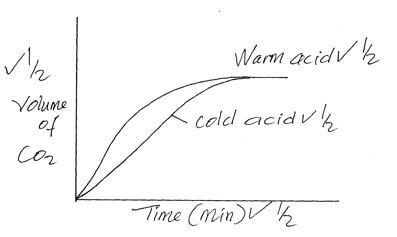
- The curves below were obtained when equal volumes of nitric (v) acid of same concentration were reacted with 25.0g of calcium carbonate, labelled Y. In one case, the acid was first warmed before the reaction.
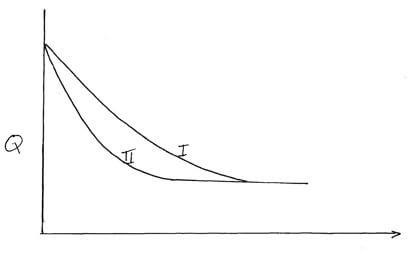
- Which curve represents the reaction involving warm nitric (v) acid? (1mk)
- Sketch the curves obtained if the graph of the volume of CO2 produced against time were plotted. (NB: on the same axis) (2mks)
-
- State two observations made when a small piece of potassium metal is put in a beaker full of water. (2mks)
- Name the group of the periodic table to which potassium belongs. (1mk)
- When a hydrocarbon with formula CxHy burns in chlorine gas, black specks and a colourless gas are obtained.
- To which homologous series does the hydrocarbon belong? (1mk)
- Write the general equation for the reaction between the hydrocarbon and chlorine gas. (1mk)
- The diagram below represents a set up for large scale manufacture of hydrochloric acid. Study it and answer the questions that follow.
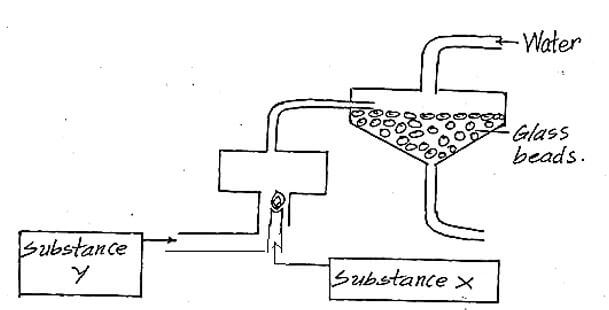
- Name the substance X. (1mk)
- What is the purpose of glass beads? (1mk)
- Give one use of hydrochloric acid. (1mk)
- When 25cm³ of 0.5M HCl is added to 25cm³ of 0.5M NaOH, the temperature of the solution rose from 23ºC to 26ºC. Given that the density of the solution is 1g/cm³ and its specific heat capacity is 4.2Jg-1k-1.
- Determine the amount of heat evolved that caused the temperature to rise. (1mk)
- Work out the molar enthalpy of neutralization for this reaction. (2mks)

MARKING SCHEME
-
- Under constant temperature and pressure, the rate of diffusion of a gas is inversely proportional to the square root of its density.√
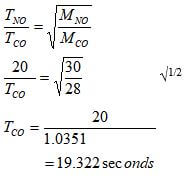
-
- A place where experiment producing poisonous gases are carried out. √1
- Storage of substances that produce foul or poisonous fumes.√1
-
- Hydrogen bond √1mk
- Covalent bond √1mk
-
-
- Ca(HCO3)2, Mg(HCO3)2
- CaSO4, MgSO4 any present √1mk
-
- Ion exchange
- Addition of sodium carbonate any present √1mk
- Distillation
-
-
- Add excess Lead metal to a certain volume of nitric(v) acid. √1/2
- Filter to obtain excess lead metal as a residue and lead (ii) nitrate as a filtrate. √1/2
- Add distilled water to sodium sulphate to form sodium sulphate solution. √1/2
- Add lead (ii) nitrate solution to sodium sulphate solution to precipitate lead (ii) sulphate and form sodium nitrate solution. √1/2
- Filter to obtain lead (ii) sulphate as a residue and sodium nitrate as a filtrate. √1/2
- Wash the residue and dry it between the filter paper. √1/2
-
- Yield of sulphur(vi) oxide decreases. Increase in temperature favours backward reaction which is endothermic. √1
- No effect on the yield.√1 Absence of a catalyst makes the equilibrium not to be achieved faster. √1
-
Empirical formula = Fe3O4√1Element
Fe
O
Mass
3.36
1.28√1
Molar mass
56
16
Mole
0.06
0.08√1
Mole ratio
1x3
1.333x3
3
4
- Reducing property√1
-
- Isotopes
-
-
-
- Yellow solution changes to pale green solution.√1
- Yellow deposit.√1
- 2FeCl3(s)+ H2S(g) → 2FeCl2(aq)+ S(s)+ 2HCl(g)
-
-
-
- A brown coating/ rust is observed on nail y. √1/2Rust occurs on Y because silver is less reactive than iron√1/2
- No brown coating/ no rust on nail X. √1/2This is because magnesium is more reactive than iron√1/2
-
- Sodium sulphite/ NaSO3√1
- Reactants Products
C-C = 348x1=348 C-C = 348x1=348
C-H = 6x414= 2484 C-H = 5x414= 2070
Cl-Cl = 243x1=243 C-Cl = 432x1=432
+ 3075KJ/mol√1 H- Cl = 340x1= 340
-3190KJ/mol√1
∆H = 3075-3190√1/2
= - 115kJ/mol√1/2 -
- Hydrogen chloride gas √1
- Polymerisation √1
- Polyvinylchloride√1
-
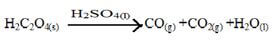
- Bubble/ pass the mixture of two gases through sodium hydroxide solution.√1
Carbon (iv) oxide is absorbed leaving carbon (ii) oxide.√1
-
- Grey solids are deposited Pb2+ ions migrate to the cathode and gain electrons to form lead metal.√1
- Electroplating
- Purification of water any one correct √1
-
- Solubility√1
- When tap is opened and closed a small drop of water dissolves a large volume √1/2 of ammonia gas creating a partial vacuum√1/2 decreasing pressure inside the flask. When the tap is opened for the second time, water gets in forming a fountain.√1
- HCl gas / NO2 gas
-
- The maximum mass in grams of a solute that saturates 100g of water at a specific temperature.
- Mass of water = 40 – 15 √1/2
= 25g of H2O√1/2
15g of salt = 25g of H2O
? = 100g of H2O
√1/2 = 60g/100g of H2O√1/2
-
- Deforestation
- More cars
- More industries
- Sea unable to absorb extra CO2 produces. Any two √√2mks
- Blue litmus paper remains blue;√1HCl gas dissolves in methylbenzene but does not dissociate to produce H+ ions. √1
-
- The activation energy should be from the reactants to the peak.√1
- The product should be below the reactants/ products should have less energy than reactants.√1
- Water has hydrogen bond√1/2 as intermolecular forces of attraction while H2S gas has weaker vanderwaal forces√1/2 of attraction between its molecules. Hydrogen bonds are stronger than weak vanderwaal forces.√1
-
- Atomic radius of R is bigger than that of G.√1
- Oxide of A is acidic while oxide of E I basic.√1
- Indicated in the periodic table before letter C.√1
-
- Curve II
-
- Melts into a silvery ball√1
Darts on the surface of water.√1
Ignites spontaneously to produce a lilac flame√1 Any two - Alkali metal
- Melts into a silvery ball√1
-
- Alkynes
- CxHy(g) + Cl2(g) → C(s) +HCl(g)
-
- Hydrogen gas
- To increase surface area for absorption.
-
- Pricking of metal.
- Treatment of sewerage
- Standardizing of pH in beers and wine. Any one √1mk
-
- ∆H = 50gx4.2Jg-1k-1 x 3K
= 630J - Moles of NaOH = 25 x 0.5 √½ = 0.0125 moles√½
1000
1 x 630 √½ = -50400J√½
0.0125
OR
Moles = -50.4kJ/mol
- ∆H = 50gx4.2Jg-1k-1 x 3K
Download Chemistry Paper 1 Questions and Answers - Cekenas Pre Mocks 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

