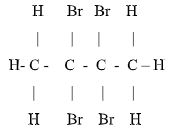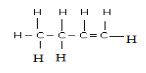Instructions to candidates
- Answer ALL the questions in the spaces provided in the question paper
- KNEC Mathematical tables and electronic calculators may be used for calculations
- All working MUST be clearly shown where necessary
- Candidates should answer the questions in English

Questions
- In the industrial preparation of oxygen, state:
- How dust particles are removed from air. (1 mark)
- Why carbon (IV) oxide is removed before the mixture is cooled to – 250C (1 mark)
- A form four student accidentally mixed Sodium Carbonate and Calcium Carbonate. Describe how he would obtain a dry sample of Sodium Carbonate from the mixture. (3 marks)
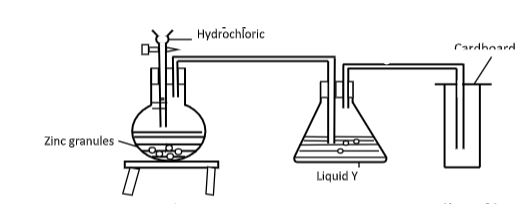
- The set up below was used to prepare dry hydrogen gas. Study it and answer the questions that follow.
- Identify a mistake in the set up (1 mark)
- Write an equation for the reaction for the reaction that produces hydrogen gas(1 mark)
- State the chemical test for hydrogen (1 mark)
- When air is bubbled through pure water (pH 7), the pH drops to 6.0.Explain (2mks)
- Explain why iron III chloride is fairly soluble in methylbenzene while Magnesium chloride is insoluble. (2 mks)
- Describe how a solid sample of Lead(II) Chloride can be prepared using the following Reagents:Dilute Nitric Acid, Dilute Hydrochloric Acid and Lead Carbonate. (3marks)
- 50cm3 of Carbon (IV) Oxide diffuses through a porous plate in 15 seconds. Calculate the time taken by 75cm3 of Nitrogen (IV) Oxide to diffuse through the same plate under similar conditions. (C = 12, 0 = 16, N = 14) (2marks)
-
- Carbon (IV) oxide is bubbled through Calcium hydroxide until there is no further change.
Explain using equations the changes observed. (2 marks) - Explain why diamond is used in cutting of glass and drilling. (1 mark)
- Carbon (IV) oxide is bubbled through Calcium hydroxide until there is no further change.
- Study the table for certain properties of substances A, B, C and D.
Melting point °C Solubility in water Electrical conduct A -119°C Soluble Solution does not conduct B 1020°C Soluble Solution conducts C 1740°C Insoluble Doesn't not conduct D 1600°C Insoluble Conducts at room temperature
Which of the substances A, B, C and D: (4 mks)- Is a metal ……………………………………………………………………………….
- Has a simple molecular structure………………………………………………………
- Has a giant ionic structure………………………………………………………………
- Has a giant covalent structure…………………………………………………………..
- A compound G reacts with 2 moles of bromine to form another compound whose structural formula is.
- What is the formula and name of compound G (2 marks)
- State the observations made when acidified potassium chromate (VI) is added to compound G (1 mark)
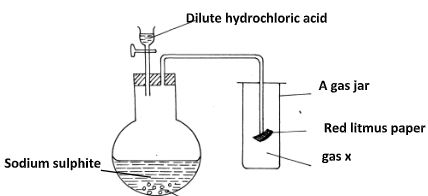
- Study the set-up below and answer the questions that follow
- Identify gas (1 mark)
- Write an equation for the reaction that produces gas x. (1 mark)
- What is the effect of the gas x above on the red-litums paper (1 mark)
- State and explain two observations made when hydrogen sulphide is bubbled through a solution containing iron (III) chloride. (2mks)
- Aluminium (III) chloride sublimes. Explain why this is possible. (2mks)
- The table below shows the solubility of a substance at various temperatures. Study it and answer the questions that follow.
Temperature(°C) Solubility in g/100g of water 0 36 40 30 80 25 110 20 - What is the meaning of solubility? (1 mark)
- What is the physical state of the substance? (1 mark)
- State and explain what would happen if a sample of a saturated solution of thesubstance at 40°C was heated to 110°C. (1mark)
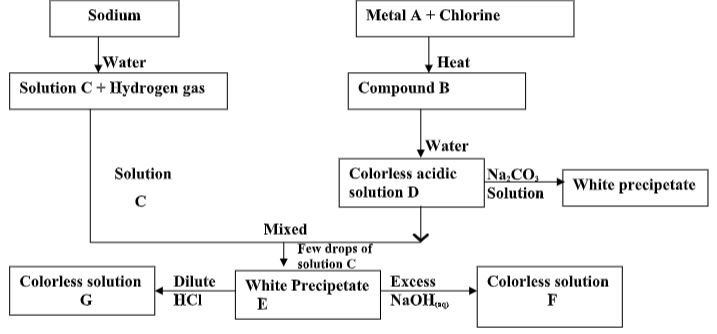
- Study the chart below and answer the questions that follow.
- Name:
- Cations present in mixture X. (1mark)
- Anions present in the solution. (1mark)
- Write an equation to show how the white precipitate in step III is formed. (1mark)
- Name:
- Study the diagram below and answer the questions
- What is the process involved in step L (1mark)
- Explain how process N and P can be affected (2marks)
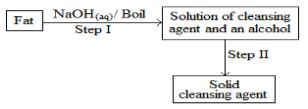
- The scheme below was used to prepare a cleansing agent. Study it and answer the questions that follow.
- Given to the type of cleansing agent prepared by the method above? (1 mark)
- Name one chemical substance added in step II (1 mark)
- What is the purpose of adding the chemical substance named in c (ii) above? (1 mark)
- Nitrates of metals A, B, C were heated and the products of the reactions recorded in the table below.
Nitrate of metal Products A Metal nitrate and oxygen B Free metal, nitrogen (IV) oxide and oxygen gas C Metal oxide, nitrogen (IV) oxide and opxygen gas - Name two possible identities of metal A. (1mk)
- Name the two possible identity of metal B (1mk)
- Calcium nitrate is one of the nitrate which forms the products in C. Using chemical equation show how the products are formed. (1mk)
- State and explain what happens to the masses of the following substances when they are separately heated in open crucibles ;(3mks)
- copper metal
- Sulphur powder
- The table below gives the first ionization energies of the alkali metals.
Element 1st ionization energy KJ mol-1 A 494 B 418 C 519 - Define the term ionization energy. (1mk)
- Which of the three metals is the least reactive? Give a reason. (2mks)
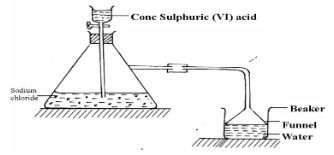
- Study the set-up below and answer questions that follow.
- Name the gas that is produced when concentrated sulphuric (VI) acid reacts with the Sodium chloride (1mark)
- Why is it necessary to use a funnel in the beaker? (1mark)
- How does the gas affect the PH of the water in the beaker? (1mark)
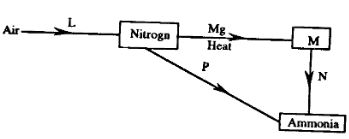
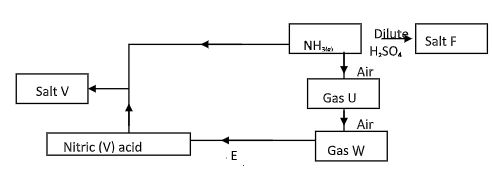
- The flow chart/diagram below outlines a method of preparing a fertilizer
- Identify U and W (1 mark)
- Give the names of salt F and V (1 mark)
- Write a balanced equation for the formation of salt F (1 mark)
-
- Draw a dot (•) and a cross (x) diagram to show bonding in Cl2O. (1 mark)
- Explain why the compound Cl2O has a very low melting and boiling point. (1 mark)
- Ethene reacts with oxygen according to the equation.
C2H4(g) + 3O2(g) → 2 CO2(g) + 2H2O(g)
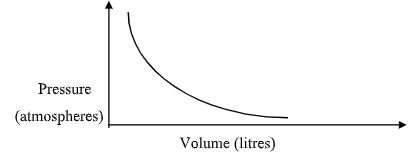
15.0 cm3 of ethene were mixed with 50cm3 of oxygen and mixture was sparked to complete the reaction. If all the volumes were measured at a pressure of one atmosphere and 250C. Calculate the volume of resulting gaseous mixture. (3 marks) - The graph below shows the behavior of a fixed mass of a gas at constant temperature.
- What is the relationship between the volume and the pressure of the gas? (1 mark)
- 3 litres of oxygen gas at 1atm atmosphere pressure were compressed to 2atm at constant temperature. Calculate the volume occupied by the oxygen gas. (2marks)
- Temporary water hardness can be removed by boiling
- What is hard water.(1 mark)
- Write a chemical equation to show how temporary hardness is removed by boiling. (1 mark)
- State one advantage of hard water. (1 mark)
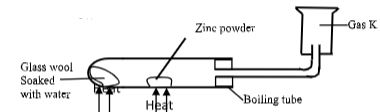
- A student set-up the experiment below to collect gas K. The glass wool was heated before heating the zinc powder.
- Why was it necessary to heat the moist glass wool before heating the zinc powder?(1 mark)
- What observation was made in the boiling tube. (1 mark)
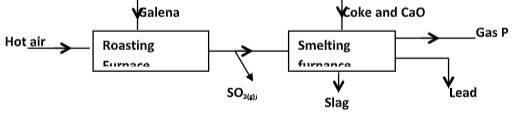
- During the extraction of lead from its ores one of the main ore used is Galena
- Write an equation for the reaction in roasting furnace. (1 mark)
- Name gas P (1 mark)
- State one use of lead metal. (1 mark)
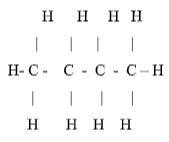
- The empirical formula of a compound is CH2 and it has a molecular mass of 42.
- What is the molecular formula of this compound? (1 mark)
- Write the general formula of the homologous series to which the compound belongs.(1mk)
- Draw the structural formula of the third member of this series and give its IUPAC name. (1mark)

Marking Scheme
-
- By passing through filters/electrostatic precipitators✓
- Carbon (IV) oxide would otherwise solidify and block the pipes✓
- Add✔ ½ water to the mixture stir ✔ ½ the mixture for all Sodium Carbonate to dissolve.
Filter ✔ ½ the mixture to obtain calcium carbonate as residue and sodium carbonate as filtrate.
Heat ✔ ½ the filtrate to evaporate ✔ ½ excess water and leave it to cool slowly for sodium carbonate to crystallize ✔ ½ . Finally filter the product and obtain pure crystals of sodium carbonate. -
- Method of gas collection is wrong,gas is lighter than air
- Zn(s) + 2HCl(aq) → ZnCl2(aq) +H2(g)
- It burns with a pop sound when ignited
- Air contains carbon (IV)oxide ;1mk this gas reacts with water to form carbonic acid hence pH drops to 6.0;1mk
- Iron III chloride is molecular and methylbenzene is also molecular while magnesium II chloride is an ionic compound
- Dissolve (✓ ½) Lead carbonate in dilute Nitric acid (✓ ½ ) React the mixture with dilute
Hydrochloric acid (1) Filter (✓ ½ ); to get Lead (II) Chloride (✓ ½ ) - 75cm3 of CO2 takes = 75 x 15/50 second ✔ ½ = 22.5 seconds✔½
Rmm of CO2 = 12 + 2 x 16 = 44 ✔ ½
Rmm of NO2 = 14 + 2 x 16 = 46 ✔ ½
TNO₂/TCO₂= √MNO₂/MCO₂
TNO2 = 22.5√46/44 seconds ✔ ½
= 23.006s ✔ ½ -
- Ca(OH)2 (aq) + CO2(s) → CaCO3(s) + H2O(l)✔ ½
Lime water forms white ✔ ½ ppt due to the formation of calcium carbonate but in excess calcium carbonate forms colourless solution due to the formation of soluble ✔ ½ calcium hydrogen carbonate.
CaCO3(s) + H2O(l) + CO2(g) → Ca(HCO3)2(aq) (2 mks) - In diamond each carbon atom is bonded to four other carbon atoms ✔ ½ arranged in a regular tetrahedron shape the whole structure of diamond extends all directions forming a rigid ✔ ½ mass of atoms. (1 mk)
- Ca(OH)2 (aq) + CO2(s) → CaCO3(s) + H2O(l)✔ ½
-
- D
- A
- B
- C
-
-
- acidified potassium chromate (VI) changes from orange to green✔ 1
-
-
- Sulphur(iv)oxide✔ 1
- Na2SO3(s) +2HCl(aq) → SO2(g) + 2NaCl(aq) + H2O(l) ✔ 1
- the red litmus paper is bleached✔ 1
- brown iron( III) ions changes to green due to reduction of iron (III) ions to iron (II) by hydrogen sulphide
- Two aluminium chloride molecules join to form a diametric molecule;1mk.the diametric molecules are held together by weak van der waals forces which easily break when heated;1mk hence the solid sublimes
-
- It is the maximum mass of solute that dissolves in 100g of water to form a saturated solution at aparticular temperature.
- it is agas
- the solution becomes more saturated with the gas
-
-
- Cu2+ , Al3+
- SO42-
- Al3+(aq) + 3OH- → Al(OH)3(s)
-
-
- fractional distillation
- N-add water
P- addition of hydrogen
-
- Soap. 1mk
- Concentrated NaCl/ Brine/ NaCl(l) 1
- To precipitate out the soap 1
-
- sodium
Potassium - silver
Mercury - 2Ca(NO3)2 → 2CaO + 4NO2 + O2
- sodium
-
- increases;1/2mk because it combines with oxygen to form the solid copper (II) oxide;1mk
- Reduces;1/2mk because it combines with oxygen to form the gaseous sulphur( IV) oxide;1/2mk which escapes;1/2mk
-
- it is the minimum amount of energy required to remove an electron from the outermost energy level of an atom in its gaseous state
- C because it requires a lot
-
- Hydrogenchloride
- it prevents sucking back/increases surface area for dissolving
- the pH of the water drops
-
- U- Nitrogen(I)oxide
W- Nitrogen(iv)oxide - F-ammonium sulphate
V-ammonium nitrate - NH3(g) + H2SO4(g) → (NH4)2SO4
- U- Nitrogen(I)oxide
-
-
- It forms a molecular structure with weak vander waals forces that are easily broken √ ½
-
- C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(g)
1 Mol 3 Mol : 2 Mole (1/2 mks)
1 Mole: 3 Vol. :2 Vol.
15cm3 :45cm3 :30 cm3
Total residual gas mixture = 5cm3 of excess oxygen + 30 cm3 of Co2
Total = 35cm3 (1/2 mks) -
- volume is inversely proportional to pressure
- P1V1 =P2V2
3 x 1 =2 x V2
V2 =1.5litres
-
- it is water that contains dissolved calcium and magnesium ions and does not lather easily
- Ca(HCO3)(aq) → CaCO3(s) +CO2(g) +H2O(l)
-
- contains calcium ions required for strong teeth
- used for brewing
- used for leather tanning
-
- to generate steam that reacts with zinc metal and also drive away air from the apparatus
- zinc glows and a yellow solid is seen
-
- 2PbS(s) +3O2(g) → 2PbO(s) +2SO2(g)
- Carbon(iv)oxide
- making lead pipes, making lead acid batteries
-
- (CH2)n = 42
(12 + 2)n = 42
14n = 42
n = 3 √ ½
MF = 3(CH2) C3H6 √ ½ - CnH2n √ 1
- But-1-ene/ √ ½/ Butene
- (CH2)n = 42
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Bunamfan Cluster Pre Mock Exam 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students