QUESTIONS
-
- In an experiment to determine the percentage of oxygen in air, the apparatus below were set up. Study the set up and the information provided to answer the questions that follow.
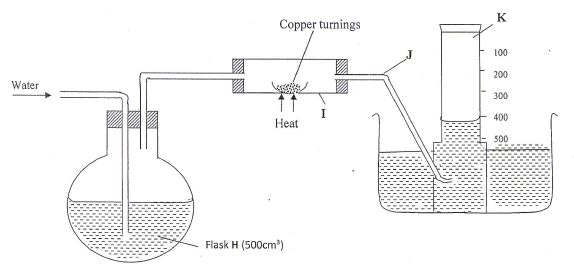
A 500cm3 measuring cylinder K was filled with water and assembled for gas collection. Copper turnings were heated red hot and water was slowly passed into 500cm3 flask H until it reached the 500cm3 mark. A colourless gas was collected in K.- What was the purpose of passing water into flask H? (1 mark)
- What observations were made in the tube I? (1 mark)
- Name one of the gases that is likely to be found in J. (1 mark)
- What was the volume of the gas collected in the measuring cylinder at the end of the experiment?(1 mark)
- Calculate the percentage of oxygen in air using the above results.(2 marks)
- Study the diagram below and answer the questions that follow.
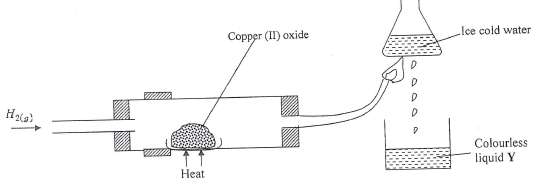
- Give one observation made in the combustion tube after some time. (1 mark)
- Write an equation for the formation of the colourless liquid Y. (1 mark)
- What was the aim of the above experiment as demonstrated in the combustion tube? Explain. (2 marks)
- In an experiment to determine the percentage of oxygen in air, the apparatus below were set up. Study the set up and the information provided to answer the questions that follow.
- Use the information below to answer the questions that follow. The letters are not the actual symbols of the elements.
Element Atomic No. M.PºC B.PºC Ionic radius (nm) P 11 98 890 0.095 Q 12 650 1110 0.065 R 12 660 2470 0.050 S 14 1410 2360 0.041 T 15 44.2 & 590 280 0.034 U 16 113 & 119 445 0.184 V 17 -101 -35 0.181 W 18 -189 -186 - -
- Write the electronic configuration of the atoms represented by letters T and W. (1 mark)
- State the nature of the oxides of the elements represented by Q and U. (2 marks)
- Why does the elements represented by the letters T and U have two values of melting points? (1 mark)
- Explain the following observations in terms of structure and bonding,
- There is an increase in boiling point from P to R. (2 marks)
- Element S has a high boiling point. (2 marks)
- There is a decrease in boiling points from U to W. (2 marks)
-
- Compare the atomic radius of U and V. Explain. (1 mark)
- Why is there no ionic radius for W reported in the table? (1 mark)
-
-
- The solubilities of potassium nitrate and potassium bromide at different temperatures wa determined. The following data was obtained.
Temperature °C 0 10 20 30 40 50 60 70 80 Solubility g/100g H2O 5 15 26 43 61 83 105 135 165 50 55 60 65 70 77 85 90 95 - Draw solubility curves for both salts on the same axis. (3 marks)
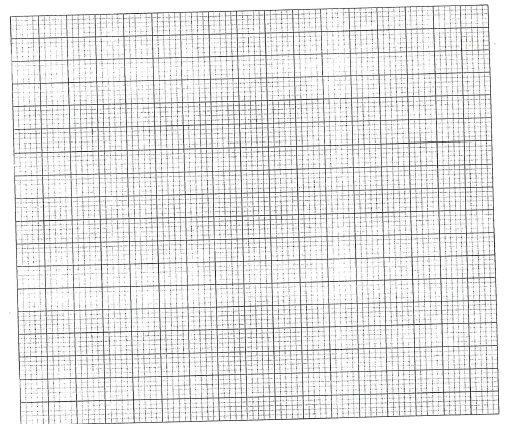
- From your graph, determine the solubility of each salt at 65°C? (1 mark)
- 100g of a saturated solution of potassium nitrate at 70°C was cooled to 20°C. What mass of the crystals will be crystallized? (2 marks)
- Draw solubility curves for both salts on the same axis. (3 marks)
- Study the flow chart below and answer the questions that follow.
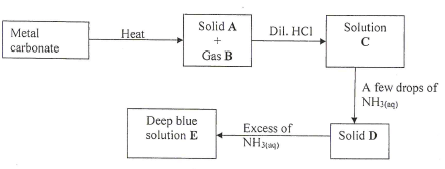
- Write an equation for the formation of solid A and gas B. (1 mark)
- Name: Solution C
Solution D (1 mark)
- Write the formula of the complex ion in solution E. (1 mark)
- The solubilities of potassium nitrate and potassium bromide at different temperatures wa determined. The following data was obtained.
- Study the flow chart below and answer the questions that follow.
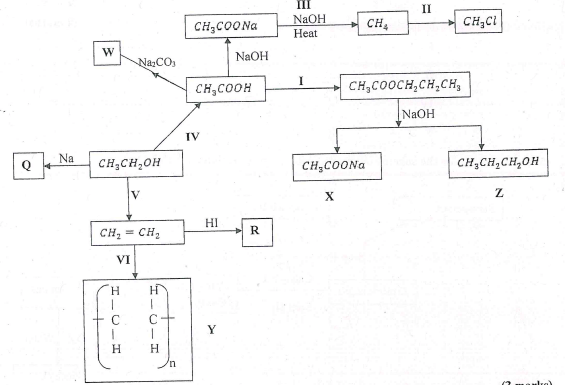
- Name substance. (3 marks)
X, Q, R - Write down an equation for the reaction represented by step III. (1 mark)
- What are the conditions and reagent required for steps?
- I - Reagent
Condition (2 marks) - IV - Reagent - ......
Condition (2 marks)
- I - Reagent
- Name the process represented by:(4 marks)
I, II, IV, V
- Name substance. (3 marks)
-
- Study the scheme below and answer the questions that follow
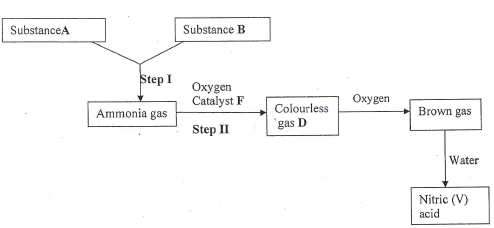
- Identify substances. (3 marks)
- State the catalyst necessary for;
Step I -
Step II - - Write an equation for the reaction taking place in step II. (1 mark)
- Write two balanced chemical equations for the reaction between chlorine gas and;
- Hot and concentrated sodium hydroxide, (1 mark)
- Dilute and cold sodium hydroxide. (1 mark)
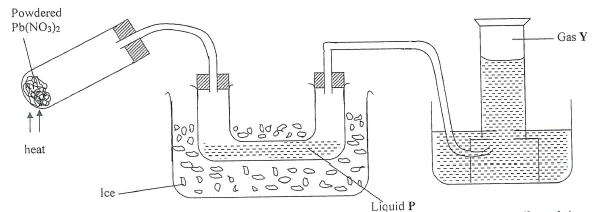
- The diagram below shows an experiment in which the Lead (II) nitrate crystals are heated.
- Name; (2 marks)
- Liquid P -
- Gas Y
- Write a balanced chemical equation for the decomposition of Lead (II) nitrate, (1 mark)
- Explain how you can distinguish between nitrogen (II)oxide and nitrogen (1) oxide. (2 marks)
- Name; (2 marks)
- Study the scheme below and answer the questions that follow
-
-
- State Hess' Law (1 mark)
- Use the equations given below to answer the questions that follow.
C2H6(g) + 7/2 O2(g) → 2CO2(g) + 3H2O(I) ΔH = -1560 kJ/mole
C(s) + O2(g) → CO2(g) ΔH = - 394 kJ/mole
H2(g) + ½O2(g)→ H2O(g) ΔH = - 286 kJ/mole- Draw an energy cycle diagram that links the enthalpy of formation of ethane to combustion of carbon, hydrogen and ethane. (1 mark)
- Determine the enthalpy of formation of ethane (2 marks)
- The diagram below shows the set-up of the apparatus used by a student to determine the enthalpy change of combustion of ethanol. The heat produced by burning fuel warms a known mass of water.
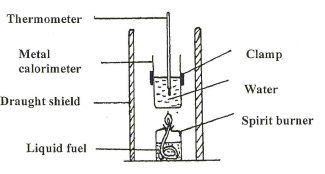
Results
Volume of water in the beaker 500cm3
Initial temperature of water 12°C
Final temperature of water = 31.5°C
Mass of ethanol burnt = 1.50g
Density of water = 1 g/cm3
Specific heat capacity= 4.2 Jg-1K-1- Calculate the heat required to raise the temperature of the water from 12°C to 31.5°C. (2 marks)
- Find the molar enthalpy of combustion of ethanol.
(C = 12, H = 1,0 = 16) - An accurate value for AHC of ethanol is -1368 kJmoll. State two sources of errors for the low figure obtained (2 marks)
- Draw an energy level diagram for the combustion of ethanol. (2 marks)
- Calculate the heating value of ethanol from the above experiment.
(C = 12, H = 1,0 = 16)
-
-
- The diagram below represents a setun of aparatus used in the electrolysis of lead (1) bromide
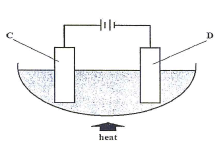
- Name electrodes C and D (1 mark)
- State and explain the observation made at electrode D (2 marks)
- Write the ionic equation for the reaction at electrode C (1 mark)
- State two applications of electrolysis (2 marks)
- The table below gives some properties of substances A, B, C, and D. Study it and answer the questions that follow.
Substance Electrical Conductivity Melting Point (°C) Boiling Point(°C) Solid Molten A Does not conduct Conducts 801 1420 B Conducts Conducts 650 1107 C Does not conduct Does not conduct 1700 2200 D Does not conduct Does not conduct 113 440 - Which particles are responsible for conductivity in substances: (2 marks)
- Which substance is likely to be silicon (IV) oxide? Explain.(2 marks)
- The diagram below represents a setun of aparatus used in the electrolysis of lead (1) bromide

MARKING SCHEME
-
-
- to displace air in flas H over hot copper turnings
- Brown copper metal changes to black copper (II) oxide
- nitrogen/ carbon (IV) oxide/ Argon/ Neon
- 390 - 400cm3
- 500 - Value above x 100%
500
-
- black copper(II) oxide changes to brown copper metal
- H2O(g) → H2O// H2+ O2 → 2H2O
- to determine/ investigate the reducing property of hydrogen. Hygrogen is above copper in the reactivity series
-
-
-
- T - 2.8.5
W - 2.8.8 - Q - Basic
U - Acidic
- T - 2.8.5
- Exhibit allotropy
-
- increase in number of decolorised electrons/ Decrease in atomic radius which in turn increases the strength of metalic bond
- A aroms are held by strong covalent bonds within the giant covalent/ atomic structure
- U,V,W have simple molecular structures in which the molecules are held by weak van der forces. There is decrease in the strength of van der waals
-
- V has a smaller atomic radius than U. V has more protons leading to excess effective nuclear charge
- stable; neither gains nor loses electrons / does not ionize
-
-
-
-
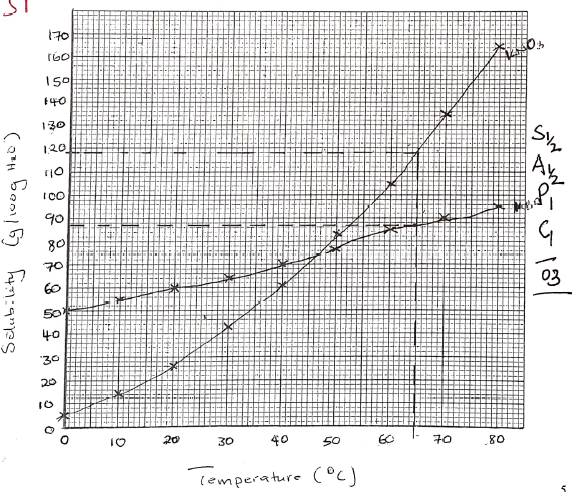
- KNO3 119g/ 100g of water 1
KBr 87g/100g of water 1 - At 70ºC
If 235g of solution contain 135g of salt
100g →100 x 135 = 57.4468g(57.45g)
235
At 20ºC
If 126g of solution → 26g of salt
100g → 100 x 26 = 20.63
126
Mass crystallised = 57.4468 = 36.81g
-
-
- CuCO3(s) →heat→ CuO(s) + CO2(g)
- C - Copper(II)chloride/ CuCl2
D - Copper (II) Hydroxide / Cu(OH)2
- [Cu(NH2)4]2+
-
-
- X - Sodium ethanate
Q - Sodium ethoxide
R - i - iodethane - CH3COONa(s) + NaOH(aq)→CH4(g) + Na2CO3(s)
-
- Reagent - Propanol
Condition - Warming/heat
Conc H2SO4(I) - Reagent - H+IK2CrO7// KMnO4(aq)
Condition - Heat// High temperature/ warm
- Reagent - Propanol
- I - esterification
II - Halogenation/chlorination
IV - Oxidation
V - Dehydration
- X - Sodium ethanate
-
-
- A - Hydrogen
B - Nitrogen
D - Nitrogen(II) oxide - Finely divided iron
Platinum - Rhodium / platinum - 4NH3(g) + 5O2(g) 4NO(g) + 6H2O(g)
-
- 6NaOH(aq) + 3Cl(g) + 3Cl2(g) → NaClO3(aq) + 5NaCl(aq) + 3H2O
- 2NaOH(aq) + Cl2(g) → NaClO(aq) + NaCl(aq)
- A - Hydrogen
-
-
- Dinitrogen tetraoxide// N2O4
- Oxygen
- 2Pb(NO3)2(s) → 2PbO(s) + 4 NO2(g) + O2(g)
- NO is odourless while N2O has a pleasant smell
N2O rekindles a glowing splint while NO does not
NO gets oxidized to NO2 while N2O does not
-
-
-
-
- Enthalpy changes when converting reactants to products is the same regardless of the route by which the chemical change occurs
-
-
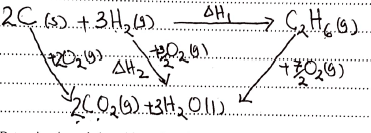
- ΔH1 = ΔH2 - ΔH3
ΔH2 = (2x - 394) + (3x - 286) = -1646
ΔH1 = -1646 + 1560 = 86kJmol
-
-
- H = mcT
= 500 x 4.2 x 19.5
= -40,950J // -40.95kJ - Moles of C2H5OH = 1.5/46 = 0.0326 moles
H = 40.95
0.0326
= 1256.13kJmol - Heat lost to the surrounding
Incorrect temperature readings
Faulty apparatus
Incomlete combustion -
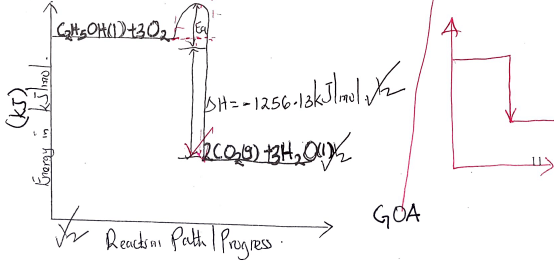
- 1256.13 = 27.31kJ-1
46
- H = mcT
-
-
-
- Anode
Cathode - Grey deposits of lead metal observed due to reduction of Pb2+ to Pb(s)
- 2Br(I) → Br2(g) + 2e-
- Purification of metals
extraction of highly reactive metals (Na & Al)
electroplating
- Anode
-
-
- mobile ions
- Delocalised electrons
- C. it does not conduct electricity in both solid and molten state and has high m.p & b.p
-
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Maranda Pre-Mock Examinations 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

