INSTRUCTIONS TO CANDIDATES
- Answer ALL questions in the spaces provided on the question paper
- You are NOT allowed to start working with the apparatus for the first 15 minutes of the 2¼ hours allowed for this paper. This time is to enable you to read the question paper and make sure you have all the apparatus and chemicals that you may need.
- All working MUST be clearly shown where necessary
- Mathematical tables and silent non-programmed electronic calculators may be used.
Question 1
You are provided with:
- Solid A – 6.2g of an alkanoic acid in a boiling tube.
- Solution B – 2.0M sodium hydroxide solution.
You are required to;- Determine the solubility of solid A at different temperatures.
- Find the molar mass of alkanoic acid.
Procedure I
- Using a burette, add 10cm3of distilled water to solid A in the boiling tube. Heat the mixture while stirring with the thermometer to about 75oC. When the entire solid has dissolved, allow the solution to cool while stirring with the thermometer. Note the temperature at which crystals of solid A appear. Record this temperature in table 1.
- Using the burette, add 2cm3of distilled water to the contents of the boiling tube. Warm the mixture while stirring with the thermometer until the solid dissolves. Allow the mixture to cool while stirring. Note the temperature at which crystals of solid A appear.
- Repeat procedure (b) two more times and record the thermometer readings in table I. Retain the contents of the boiling tube for use in procedure II.
- Complete table 1 by calculating the solubility of solid A at different temperatures.
Table 1
(5 marks)Volume of water added(cm3)
Temperature at which first crystals appear (oC)
Solubility of solid A (g/100g of water)
10
12
14
16
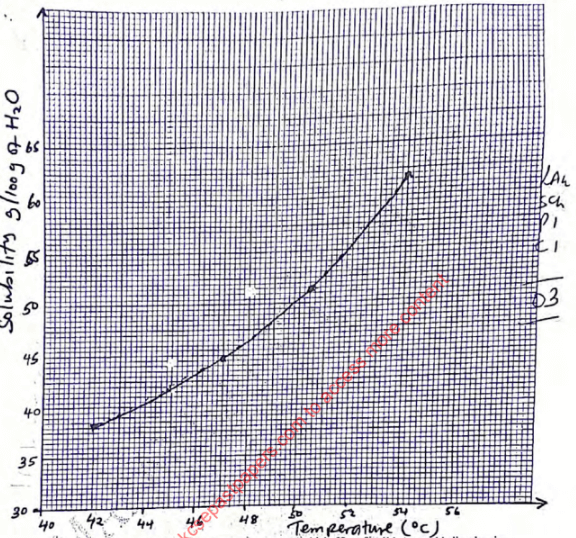
- On the grid provided, plot a graph of the solubility of solid A against temperature. (3 marks)
- Using the graph determine the temperature at which 52g of solid A would dissolve in 100cm3of water. (1 mark)
- Complete table 1 by calculating the solubility of solid A at different temperatures.
Procedure II
- Transfer the contents of the boiling tube in procedure I into a 250ml volumetric flask. Rinse both the boiling tube and the thermometer with distilled water and add it to the volumetric flask. Add more distilled water to make up to the mark. Transfer the solution into a 250ml beaker. Label this solution E. Rinse the volumetric flask with distilled water ready for use in step (ii).
- Using measuring cylinder, place 25cm3of solution B into a 250ml volumetric flask. Add about 200cm3of distilled water and shake well. Add more distilled water to make up to the mark. Label this solution F.
- Fill the burette with solution E. using a pipette filler, place 25cm3of solution F into a conical flask. Add 2-3 drops of phenolphthalein indicator and titrate with solution E. record your results in table II. Repeat this procedure two more times to complete the table 2 below.
Table 2
(4 marks)1
2
3
Final burette reading (cm³)
Initial burette reading (cm³)
Volume of Solution E used (cm³)
- Determine the average volume of solution E used. (1 mark)
- Determine the concentration of solution F in moles per litre. (1 mark)
- Calculate the number of moles in 25cm3of solution F. (1 mark)
- Calculate the moles of alkanoic acid, solution E used (1 mole of acid reacts with 2 moles of base). (1 mark)
- Calculate the concentration of solution E in moles per litre. (1 mark)
- Determine the relative formula mass of the alkanoic acid, Solid A. (C =12, H=1, O=16)
(2 marks)
Question 2
You are provided with solid R. Carry out the tests below. Write your observations and inferences in the spaces provided.
Place solid R in a boiling tube, add 10cm3of distilled water and shake well. Use about 2cm3portions for test (i) to (v) below.
- Add 3 drops of sodium sulphate solution.
Observations Inferences (1 mark) (1 mark) - Add sodium hydroxide solution dropwise until in excess.
Observations Inferences (1 mark) (1 mark) - Add aqueous ammonia dropwise until in excess.
Observations Inferences (1 mark) (1 mark) - Add 3 drops of barium (II) nitrate solution
Observations Inferences (1 mark) (1 mark) - Add 3 drops of potasium dichromate(VI) solution
Observations Inferences (1 mark) (1 mark)
Question 3
You are provided with an organic substance, solid Q. You are required to carry out the tests indicated below.
Place a ALL of solid Q in a boiling tube. Add about 10 cm3of distilled water and shake well. Divide the mixture into four equal portions in test tubes.
| Observations | Inferences |
| (1 mark) | (1 mark) |
- To the first portion, add two drops of acidified potassium manganate (VII) solution.
Observations Inferences (1 mark) (1 mark) - To the second portion, add three drops of acidified potassium dichromate (VI).
Observations Inferences (1 mark) (1 mark) - To the third portion, add all the sodium hydrogen carbonate.
Observations Inferences (1 mark) (1 mark) - Test the pH of the fourth portion using universal indicator solution provided.
Observations Inferences (1 mark) (1 mark)
CONFIDENTIAL
INSTRUCTIONS TO SCHOOLS
In addition to the fittings and apparatus found in a chemistry laboratory, each candidate will require the following:
- Each candidate
- About 50cm3of solution B
- One burette 0 – 50 ml
- One pipette 25.0ml and a pipette filler
- One Filter funnel
- 250ml beaker
- 6.2g of Solid A exactly weighed in a stoppered container
- Two clean dry 250 ml conical flasks
- Five (5) clean and dry test tubes on a test tube rack
- One boiling tube
- Thermometer (-10oC to 110oC)
- 0.2g of solid sodium hydrogen carbonate supplied in a stoppered container
- About 500cm3of distilled water supplied in a wash bottle
- One 250ml volumetric flask
- One 50ml measuring cylinder
- Two (2) labels
- One metallic spatula
- One test tube holder
- pH chart
- A white tile
- About 0.5g of solid R supplied in a stoppered container
- About 0.5g of solid Q supplied in a stoppered container
- Access to:
- Phenolphthalein indicator supplied with a dropper
- Bunsen burner
- 2.0M aqueous ammonia supplied with a dropper
- 2.0M sodium hydroxide solution supplied with a dropper
- Acidified potasium dichromate (VI) solution supplied with a dropper
- Acidified potassium manganate (VII) solution supplied with a dropper 7. 1.0M Sodium sulphate solution supplied with a dropper
- 0.5M Barium (II) nitrate solution supplied with a dropper
- Universal indicator solution
- Preparation of solutions and solids
- Solution B (2.0M Sodium Hydroxide) is prepared by dissolving 80.0g of sodium hydroxide in 700cm3of distilled water and diluting it to one litre.
- Solid R is Zinc Sulphite
- Solid Q is Maleic acid
- Solid A is Exactly 6.2g oxalic acid
MARKING SCHEME
Question 1
Procedure I
- Table 1
Volume of water added(cm3)
Temperature at which first crystals appear (oC)
Solubility of solid A (g/100g of water)
10
54.0
62.0
12
50.5
51.7
14
47.0
44.3
16
42.0
38.8
-
Procedure II
Table 2
|
|
1 |
2 |
3 |
|
Final burette reading (cm³) |
12.6 |
12.7 |
12.8 |
|
Initial burette reading (cm³) |
0.0 |
0.0 |
0.0 |
|
Volume of Solution E used (cm³) |
12.6 |
12.7 |
12.8 |
- = 12.6 + 12.7 + 12.8
3
= 12.7cm3 - 2 × 25 = M × 250
M = 0.2M - 0.2 × 25 = 0.005
1000 - 0.005 = 0.0025
2 - 0.0025 × 1000 = 0.19685
12.7 - Conc. in g/l = 6.2 × 1000
250
= 24.8
RFM = 24.8.
0.19685
= 125.98
Question 2
-
Observations Inferences No white ppt (1 mark) Ca2+, Pb2+, Ba2+ absent. (1 mark) -
Observations Inferences White ppt that dissolves in excess (1 mark) Zn2+ , Al3+ present (1 mark)
Penalize ½mk mfor Pb2+ if mentioned -
Observations Inferences White ppt soluble in excess (1 mark) Zn2+ present (1 mark)
Penalize FULLY for any contradicting ion mentioned -
Observations Inferences White ppt (1 mark) SO42−, SO32− present (1 mark)
Penalize ½mk for CO32− if mentioned -
Observations Inferences Orange Cr2O72−
Solution changes to green (1 mark)SO32− present (1 mark)
Penalize FULLY for any contradiction
Question 3
| Observations | Inferences |
| Dissolves to form a colourless solution (1 mark) | Polar compound (1 mark) |
-
Observations Inferences Purple KMnO4 changes to colourless/ is decolourised (1 mark)  present (1 mark)
present (1 mark) -
Observations Inferences K2Cr2O7 remains orange (1 mark) 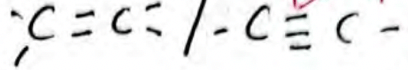 present
present
or
R−OH absent (1 mark) -
Observations Inferences Effervescence/ Bubbles/ Fizzling (1 mark) R–COOH present
accept H+/H3O+ for ½. (1 mark) -
Observations Inferences pH value 1/2/3 (1 mark)
Value must be specifiedstrongly acidic (1 mark)
Download Chemistry Paper 3 Questions and Answers - Maranda High Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
