Instructions to Candidates:
- Answer ALL questions
- ALL working must be shown clearly where necessary.
- Mathematical tables and silent non-programmable calculators may be used.
For Examiner’s Use Only
|
Questions |
Maximum Score |
Candidate’s Score |
|
1 -29 |
80 |
|

QUESTIONS
- The electron arrangement of ions X+, Y2+ and W3- are 2.8, 2.8 and 2.8.8 respectively.
- Write the electron arrangement of their atoms. (1½ marks)
- Arrange the atoms in the order of increasing atomic radius starting with the smallest. Give a reason for the order. (1½ marks)
- The diagram below shows a Bunsen burner when in use.
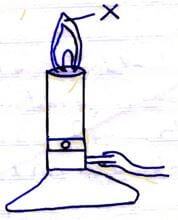
- State the condition under which the Bunsen burner produces the flame shown in the diagram above. (1 mark)
- Describe an experiment that can be carried out to confirm that the region labeled X is the hottest. (2 marks)
-
- Chlorides of Sodium and aluminium are given in the table below. Complete the table by writing the properties of the chlorides. (2 marks)
Property
NaCl
AlCl3
Bonding
Structure
- Sodium carbonate powder were added to aqueous solution of aluminium chloride. State and explain the observation made. (1 mark)
- Chlorides of Sodium and aluminium are given in the table below. Complete the table by writing the properties of the chlorides. (2 marks)
-
- Explain why molten Magnesium Chloride conducts electric current while sugar solution do not. (1 mark)
- Complete the table below by writing the observations, anode and cathode half-equations for electrolysis of molten Lead (II) Chloride. (2 marks)
Anode
Cathode
Observations
Half-equations
- The set-up below was used during a class experiment. Study it and answer the questions that follow.
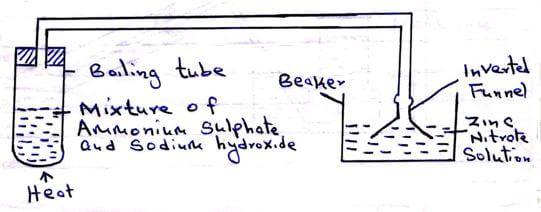
- Identify the gas produced in the boiling tube. (1 mark)
- State and explain the observation made in the beaker. (2 marks)
- The following data refers to element X.
Calculate the relative atomic mass of X. (2 marks)Isotope
X1
X2
X3
Mass of isotope
54
56
57
% abundance
6
92
2
-
- State the Charles’ Law (1 mark)
- Using Kinetic Theory, explain why the pressure of a fixed mass of a gas decreases with increase in volume at a constant temperature. (2 marks)
- The set up below was used to prepare a sample of methane gas. Study it and answer the questions that follow.
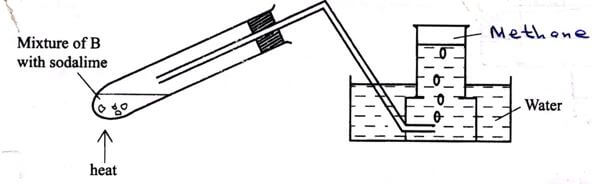
- Identify substance B (1 mark)
-
- Give one condition that is necessary for methane to react with chlorine gas. (1 mark)
- Write an equation for the reaction that occurs when methane react with excess chlorine gas. (1 mark)
- Chemical tests were carried out on separate samples of water from the same river. The observations made were recorded as shown in the table below.
Test
Observation
(i)
Addition of few drops of barium chloride
White precipitate formed
(ii)
Addition of sodium hydroxide dropwise until in excess
White precipitate dissolves
(iii)
Addition of aqueous ammonia until in excess
White precipitate insoluble
(iv)
Addition of acidified barium chloride
White precipitate
- State the inference in;
Test (i) . (1 mark)
Test (ii) (1 mark) - Identify the cation and anion present in the sample of water. (1 mark)
- State the inference in;
- 2M potassium hydroxide has higher pH value as compared to 2M ammonia solution. Explain. (2 marks)
-
- Name two reagents used to prepare hydrogen sulphide gas. (1 mark)
- Hydrogen Sulphide and Sulphur (IV) oxide were separately bubbled into acidified potassium manganite (VII) solution. State and explain the observation made in each case. (2 marks)
- Hydrogen sulphide
- Sulphur (IV) oxide
- An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 2.58g of Y on complete combustion produced 5.28g of carbon (IV) oxide and 1.62g of water. Determine the empirical formula of Y. (C = 12.0 H = 1.0 O = 16.0) (3 marks)
- The set-up below was used to investigate the properties of ammonia gas.
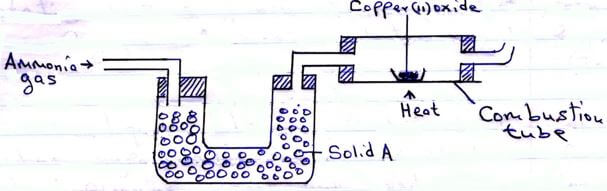
- Identify solid A. (1 mark)
- State
- The observation made in the combustion tube. (1 mark)
- Property of ammonia gas shown in this experiment. (1 mark)
- Study the flow chart below and answer the questions that follow.
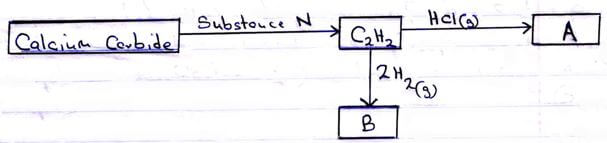
- Identify substance N (1 mark)
- Name substance B (1 mark)
-
- Draw the structural formula of substance A. (½ mark)
- Draw one repeat unit of polymer formed by substance A. (½ mark)
- Starting with a piece of sodium metal, describe how crystals of sodium nitrate may be prepared. (3 marks)
-
- Common liquid bleaches contain solution of sodium hypochlorite is formed when chlorine react with sodium hydroxide solution.
- Give two conditions under which sodium hypochlorite is formed. (1 mark)
- Explain how sodium hypochlorite works as a bleaching agent. (1 mark)
- Describe a test for hydrogen chloride gas. (1 mark)
- Common liquid bleaches contain solution of sodium hypochlorite is formed when chlorine react with sodium hydroxide solution.
- Draw a well labeled diagram of a set-up that can be used to prepare a dry sample of carbon (IV) oxide gas using marble chips. (3 marks)
- 6.84g of aluminium sulphate were dissolved in 400cm3 of water. Determine the number of sulphate ions in the solution.
- Burning magnesium and a burning splint were separately introduced into a gas jar full of carbon (IV) oxide. State and explain the observations made.
- Burning Magnesium (2 marks)
- Burning splint. (1 mark)
- Using dot (•) and cross (X) diagram, show bonding in the following substances.
- Water molecule (1 mark)
- Hydroxonium ion (H3O+) (1 mark)
- Give a reason why water molecule can combine with hydrogen ion. (1 mark)
- Describe how you can obtain zinc sulphate crystals from zinc sulphate solutions. (1 mark)
- 120cm3 of ethane were mixed with 40cm3 of oxygen and the mixture exploded to complete reaction. Calculate the volumes of the resulting gaseous mixture when measured at room temperature and pressure. (3 marks)
- In an experiment to study properties of carbon, a mixture of concentrated nitric (IV) acid and wood charcoal was heated in a boiling tube. State and explain the observation made. (2 marks)
-
- Write formulas of two substances that causes temporary hardness in water. (1 mark)
- Give one advantage of hard water in brewing industry. (1 mark)
- Write an equation to show how boiling removes hardness of water. (1 mark)
- Clean magnesium ribbon was dropped into a solution of hydrogen chloride gas in methylbenzene.
- State and explain the observations made. (1 mark)
- The experiment was repeated using solution of hydrogen chloride in water. State and explain the observation made. (1 mark)
-
- Define molar heat of solution. (1 mark)
- 1.0g of zinc powder was added to 50cm3 of 0.2m copper (II) sulphate solution and the mixture stirred gently. The temperature of the mixture rose from 20ºC to 27ºC. Calculate the molar heat of displacement of copper (specific heat capacity = 4.2KJ/Kg/K, density of solution = 1g cm-3) (2 marks)
- The following equation represents the reaction that occurs during contact process.
2SO2(g) + O2 (g) ↔ 2SO3(g)- Name the catalyst used in this reaction (1 mark)
- The sulphur (VI) oxide is normally absorbed in concentrated sulphuric (VI) acid and not in water. Explain. (1 mark)
-
- When ice is heated, temperature remains constant at 00C until all the ice has melted. Explain this explanation. (1 mark)
- The scheme below shows the energy changes that are involved between ice, water and steam. Study it and answer the questions that follow.
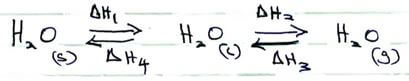
- What name is given to the energy change ΔH2 (1 mark)
- What is the sign of ΔH4 (1 mark)
- The following results were obtained during an experiment to determine the solubility of potassium chlorate (V) in water at 30ºC.
Mass of evaporating dish = 15.86g.
Mass of evaporating dish + saturated solution at 30ºC = 26.8g.
Mass of evaporating dish + solid potassium chlorate (V) after evaporating to dryness = 16.86g.
Calculate the mass of the saturated solution containing 60.0g of water at 30ºC. (3 marks)

MARKING SCHEME
-
- X 2.8.1 √½
Y 2.8.2 √½
W 2.8.5 √½ - W Y X √½
Atomic radius decrease with increase in number of protons √1 /nuclear charge
- X 2.8.1 √½
-
- When the air hole is open √1
- Slip a white manila paper or wooden splint √½ and quickly √½ remove before it catches fire. The middle part burns uniformly. √1
Accept the diagram which is well labeled.
-
- Property Nacl Alcl3
Bond ionic √½ covalent √½
Structure giant ionic √½ molecular √½ - There is effervescence √½ Aluminium chloride hydrolyses in water forming acidic solution. √½
- Property Nacl Alcl3
-
- Molten magnesium chloride has mobile ions √½ while sugar solution has molecules √½ /lack mobile ions.
-
Penalize ½ for missing or wrong stare symbols.Anode
Cathode
Observations
Green-yellow gas
Grey solid
Half-equations
2Cl(l) → Cl2(g) + 2e
Pb2+(l) + 2e → Pb(s)
-
- Ammonia gas/NH3(g) √1
- White precipitate formed that dissolves in excess. √1
Ammonia gas dissolves in aqueous solution to form ammonium hydroxide √½ which react with zinc ions forming zinc hydroxide √½ that dissolves in excess to form tetra ammine zinc (II) ion.
- ((54 x 6)+(56x 92)+(57 x 2))/100 √1
= 55.9 √1
No units
Penalize fully when units are shown. -
- The volume of a fixed mass of a gas is directly proportional to its absolute temperature at constant pressure. √1
- Increase in volume reduces the number √1. Collisions of gas molecules and the walls of the container causing a decrease in pressure. √1
-
- Sodium ethanoate √1/CH3COONa
-
- Ultra-violet light /sunlight.√1
- CH4(g) + 4Cl2(g) → CCl4(g) + 4Hcl(g)√1
-
- Test (i) SO2-3, SO2-4 and CO2-3 √1 present. at least two
Test (iii) Al3+ √1 only penalize Pb2+ - Al3+ √½ penalize Pb2+
SO2-4 √½
- Test (i) SO2-3, SO2-4 and CO2-3 √1 present. at least two
- Potassium hydroxide is a strong base √½ and dissociate /ionizes fully √½ giving more OH- ion √½
Ammonia is a weak base √½ hence ionizes partially in water. -
- Iron (II) sulphide
Dilute hydrochloric acid mark as a pair for 1 mark.
NB: Any sulphide and dilute acid. - Hydrogen sulphide
Acidified potassium manganite (VII) is decoloured √½ and yellow deposit. √1
Sulphur (IV) oxide
Acidified potassium manganite (VII) is decolourised. √½
- Iron (II) sulphide
- Mass of carbon in CO2 = 12/44 x 5.28 = 1.44g √½
Mass of hydrogen in H2O = 2/18 x 1.62 = 0.188 √½
Mass of oxygen = 2.58g – (1.44 + 0.18) = 0.96g √½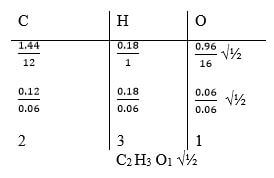
C2 H3 O1 √½ -
- Calcium oxide √1 Reject any other
-
- Black Copper (II) oxide changes to brown copper metal √1
- Reducing agent √1
-
- Water √1
- Ethane √1

Drop a piece of sodium metal in distilled water √1 in a beaker, to the resulting solution add dilute nitric (IV) acid. √½ Evaporate √½ the resulting mixture to saturation and cool √½ for crystals to form, dry √½ crystals between filter papers.-
-
- Cold √½ and dilute √½ sodium hydroxide.
- Sodium hypochlorite (NaOCl) dissociates √½ giving out nascent oxygen √½ to the dye causing it to bleach.
- Introduce a glass rod dipped in concentrated ammonia solution, √½ white fumes formed. √½
-
-
- Moles of Al2 (SO4)3 sulphate = 6.84/342 = 0.02 moles √½
Molarity = (0.02 x 1000)/400 = 0.05m √½
Al2 (SO4)3(a) 2 Al3+(aq)) + 3 SO42-09 √½
Molarity of SO2-4 = 0.05 x 3 = 0.15m √½
Number of SO2-4 = 0.15 x 6.0 x 1023 √½
= 9.0 x 1022 ions √½
Accept alternative method - Burning magnesium √
Continues to burn, √½ a mixture of white solid and black specks formed. √½ Heat √½ produced decomposes carbon (IV) oxide to carbon and oxygen. √½
Burning splint
It extinguished/put off √½ carbon (IV) oxide does not support combustion. √½ -
-
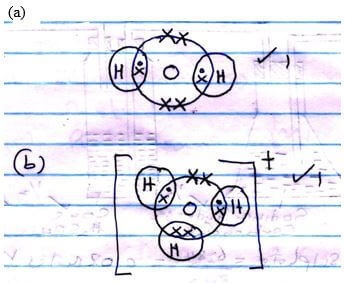
- Water molecule has lone pair of electrons which it can dissolve to H+ √1
- Heat zinc sulphate to saturation √½ and allow saturated solution to cool √½ for crystals to form.
- 2 C2H6(g) + 5 O2(g) 4 CO2(g) + 6 H2O(l) √1
Volume of Ethane burned = (2 x 40)/5 = 16cm3 √½
Volume of ethane remaining = 120 – 16 = 104cm3√½
Volume of carbon (IV) oxide formed = (4 x 40)/5 = 32cm3 - Brown fumes evolved, √1 carbon reduces nitric (V) acid to Nitrogen (IV) oxide √½ and water and carbon is oxidized to carbon (IV) oxide √½
-
- Mg(H CO3)2 √½
Ca (HCO3)2 √½ - Contain calcium ions √1 required in bone formation and strengthening of teeth/ improve taste.
- Mg(H CO3)2(aq) → Mg CO3(s) + CO2(g) + H2O(l)
Or
Ca (HCO3)2(aq) → COCO3(s) + CO2(g) + H2O(l)
Penalize ½ mark for missing or wrong symbol.
- Mg(H CO3)2 √½
-
- No effervescence /No bubbles √½
Hydrogen chloride gas in methylbenzene is not ionized. √½ - Effervescence/ Bubbles of gas √½
Hydrogen chloride gas in methylbenzene ionizes and it is acidic. √½
- No effervescence /No bubbles √½
-
- It is the heat change when one mole of a substance dissolves in water to form infinitely dilute solution / very dilute. √1
- Heat change = 50/1000 x 4.2 kJ/Kg/K x 7 √½
= 1.47KJ √½
Moles of copper (II) sulphate = (50 x 0.2 )/1000 √½
= 0.01 moles √½
Molar heat of displacement of Copper (II) ions
(1 x 1.47)/0.01 √½ = -147 KJ/mole√½- ΔH(-) sign must be shown and units must be correct.
- Penalize fully if missing.
-
- Vanadium (V) oxide √1 or Platinum
- Reaction between Sulphur (VI) oxide and water is highly exothermic √1 and hence boil the acid
-
- Heat absorbed is used to weaken the forces √1 of attraction between the particles resulting in change of state.
Accept intermolecular forces. -
- Latent heat of vaporization √½
- It is negative √1
- Heat absorbed is used to weaken the forces √1 of attraction between the particles resulting in change of state.
- Mass of saturated solution = (26.8g – 15.86g)
= 10.94g √½
Mass of a solute = (16.86 – 15.86)g
= 1g √½
Mass of solvent = 10.94g – 1g
= 9.94g √½
If 1g → 9.94g of water
? → 60g of water
=((60 x 1)/9.94) = 6.03g √½
Mass of saturated solution = (60 + 6.03)g √½
= 66.03g √½
Download Chemistry Paper 1 Questions and Answers - Arise and Shine Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
