INSTRUCTION:
- ANSWER ALL THE QUESTIONS IN THE SPACES PROVIDED
- Define the following terms (4marks)
- Drug
- Prescription
- Nekesa visited a hospital and was given a syrup whose prescription was2×3.How should she take the syrup? 2marks)
-
- Explain the following:
- It is always advisable to scoop chemical substances using a clean spatula. (1mark)
- Flammable substances should always be kept away from flames in the laboratory. (1mark)
-
- Give three differences between luminous and non-luminous flames. (3 marks)
- How is the non-luminous flame produced? (1 mark)
-
- Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice cream venders. (1 mark)
- Name one piece apparatus used to measure volume of gases. (1 mark)
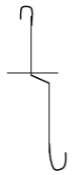
- Draw a diagram of a deflagrating spoon and state its use (2 marks)
- Explain the following:
- Define the following terms (5marks)
- Solute.
- Solvent.
- Solution.
- Residue.
- Filtrate.
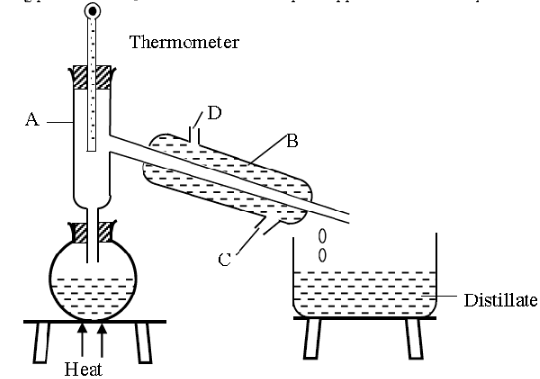
- A form one had a mixture of ethanol and water. Ethanol has a boiling point of 78°C while water has a boiling point of 100°C. The student then set up the apparatus below to separate the mixture.
- Name the piece of apparatus labelled B. (1 mark)
- What is the purpose of the thermometer in the set up? (1 mark)
- Name the part labelled A and state its function (2 marks)
- Which liquid was collected first? Explain (2 marks)
- What is the name given to the above method of separating mixtures?
(1 mark) - What passes through parts Cand D? (2marks)
- What property of the components of the mixture makes it possible for the components to be separated by the method? (1mark)
- State one applications of the above method of separation. (1 marks)
- Describe how you can extract oil from ground nuts? (3marks)
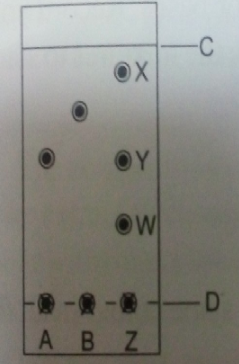
- Spots of pure pigments A and B, and a mixture of Z were placed on a filter paper and allowed to dry. The paper was then dipped in a solvent. The results obtained were as on the chromatogram.
- which is the ;( 2marks)
- Base line?
- Solvent front?
- Which of the pure pigments was a component of Z Explain? (2marks)
-
- Name a solvent that is used in paper chromatography. (1mark)
- Why is water not a suitable solvent in paper chromatography? (2marks
- Give 2 applications of chromatography in our daily lives? (2marks)
- which is the ;( 2marks)
- Explain the differences between solid, liquid and gaseous states using the theoretical model (diagram) of matter in terms of the kinetic theory (6marks)
- Define the following terms (4marks)
- An atom
- A molecule
- An element
- A compound
- Complete the following table (4marks)
Element Symbol Potassium Na Silver Au Iron Pb Copper Mercury - Name the elements present in the following compounds.
- Sodium Bromide (2marks)
- Magnesium nitride (2marks)
- Write a word equation for the reaction between:
- Carbon and oxygen (2marks)
- Sodium and sulphur (2marks)
- Give three differences between a temporary chemical change and a permanent chemical change (3marks)

MARKING SCHEME
- Define the following terms (4marks)
- Drug
- A drug is any substance, natural or manufactured which when used alters the way the body functions
- Prescription
- The written instructions by a qualified medical officer, giving details on type of drugs and how the drugs should be taken
- Nekesa visited a hospital and was given a syrup whose prescription was2×3.How should she take the syrup? 2marks)
- She should take 2 teaspoons after every 8 hours in a day.
- Drug
- Explain the following:
-
- It is always advisable to scoop chemical substances using a clean spatula. (1mark)
- To prevent contamination of the chemicals.
- Flammable substances should always be kept away from flames in the laboratory. (1mark)
- To prevent fire accidents.
- It is always advisable to scoop chemical substances using a clean spatula. (1mark)
-
- Give three differences between luminous and non-luminous flames. (3 marks)
Luminous Non-luminous -Bright yellow flame
-Sooty
-4-regions
-Large and wavy-Blue flame
-Non-sooty
-3-regions
-Short and steady - How is the non-luminous flame produced? (1 mark)
- When the air hole is fully open.
- Give three differences between luminous and non-luminous flames. (3 marks)
-
- Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice cream venders. (1 mark)
- Sublimes leaving no wetness
- Name one piece apparatus used to measure volume of gases. (1 mark)
- Syringe
- Draw a diagram of a deflagrating spoon and state its use (2 marks)
It is used for holding substances being burned
- Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice cream venders. (1 mark)
-
- Define the following terms (5marks)
- Solute. This is a substance that dissolves in a liquid
- Solvent. This is a liquid that dissolves a solute
- Solution. This is the resulting mixture of solute and solvent
- Residue. The solid particles that are left on the filter paper
- Filtrate. The liquid that goes through the filter paper
-
- A form one had a mixture of ethanol and water. Ethanol has a boiling point of 78°C while water has a boiling point of 100°C. The student then set up the apparatus below to separate the mixture.
- Name the piece of apparatus labelled B. (1 mark)
- Liebig condenser ✓ 1
- What is the purpose of the thermometer in the set up? (1 mark)
- It helps in determining ✓ 1 the boiling range so that pure ethanol is obtained. When liquids boil, the temperatures remain constant.
- Name the part labelled A and state its function (2 marks)
- Part A: Fractionating column. ✓ 1
- It increases the surface area for condensation of the substance whose boiling point has not been reached. ✓ 1 or
- Provides a surface in which vapour condenses into liquid and the liquid redistills. ✓ 1
- Which liquid was collected first? Explain (2 marks)
- Ethanol was collected first ✓ 1
- It has a lower boiling point 780C, hence it boils first. ✓ 1
- What is the name given to the above method of separating mixtures? (1 mark)
- Fractional distillation ✓ 1
- What passes through parts Cand D? (2marks)
C…..cold water in
D…..Hot water out - What property of the components of the mixture makes it possible for the components to be separated by the method? (1mark)
- Have different but close boiling points. ✓ 1
- State one applications of the above method of separation. (1 marks)
- Manufacture of spirits
- Fractional distillation of crude oil.
- Obtaining oxygen from liquid air. (Any 0ne which are correct (1 mark)
- Name the piece of apparatus labelled B. (1 mark)
- A form one had a mixture of ethanol and water. Ethanol has a boiling point of 78°C while water has a boiling point of 100°C. The student then set up the apparatus below to separate the mixture.
- Describe how you can extract oil from ground nuts? (3marks)
- Using pestle and mortar crush groundnut seeds while adding propanone a little at a time. Decant the resulting solution into an evaporating basin. Leave the solution in the sun for some time.
- Spots of pure pigments A and B, and a mixture of Z were placed on a filter paper and allowed to dry. The paper was then dipped in a solvent. The results obtained were as on the chromatogram.
- which is the ;( 2marks)
- Base line?
- D
- Solvent front?
- C
- Base line?
- Which of the pure pigments was a component of Z Explain? (2marks)
- A.This is because it moves the same distance as a component Y which is a pigment of Z
-
- Name a solvent that is used in paper chromatography. (1mark)
- Propanone
- Why is water not a suitable solvent in paper chromatography? (2marks)
- This is because it cannot dissolve various coloured pigments and also it does not spread but only causes wetness on the filter paper
- Give 2 applications of chromatography in our daily lives? (2marks)
- In sports, to identify banned substances e.g steroids in blood sample
- In the pharmaceutical industry, to test purity of drugs.
- In food industry to identify contaminants in food and drinks.
- In the cosmetics industry, to identify harmful substances.
- Name a solvent that is used in paper chromatography. (1mark)
- which is the ;( 2marks)
- Explain the differences between solid, liquid and gaseous states using the theoretical model (diagram) of matter in terms of the kinetic theory (6marks)
- In the solid state, the particles are closely packed together and can only vibrate within fixed positions. They do not move from one point to another because they are held together by forces. In the liquid state, particles are not as close together as they are in solid state. They can move from one position to another within the liquid. In gaseous state the particles are far apart and free to move more randomly in all directions. They have the highest kinetic energy
- Define the following terms (4marks)
- An atom
- An atom is the smallest particle of an element that can take part in a chemical reaction
- A molecule
- A molecule is the smallest particle of an element or compound which can exist separately
- An element
- An element is a pure substance which cannot be split into simpler substances by chemical means.
- A compound
- A compound is pure substance made up of two or more elements chemically combined.
- An atom
- Complete the following table(4marks)
Element Symbol Potassium k Sodium Na Silver Ag Gold Au Iron Fe Lead Pb Copper Cu Mercury Hg - Name the elements present in the following compounds.
- Sodium Bromide (2marks)
- Sodium and Bromine
- Magnesium nitride (2marks)
- Magnesium and Nitrogen
- Sodium Bromide (2marks)
- Write a word equation for the reaction between:
- Carbon and oxygen (2marks)
Carbon+Oxygen→Carbon (IV) Oxide - Sodium and sulphur (2marks)
Sodium + Sulphur→Sodium sulphide
- Carbon and oxygen (2marks)
- Give three differences between a temporary chemical change and a permanent chemical change(3marks)
Temporary chemical change Permanent chemical change A new substance is formed No new substance is formed Heat energy is not evolved or absorbed Heat energy is released or absorbed The change is irreversible The change is irreversible
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions And Answers - Form 1 Term 2 Opener 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students