PHYSICS
FORM 1 OPENER EXAM
TERM 3 2022
INSTRUCTION TO CANDIDATES:
- Write your name, Admission number and class in the spaces provided above.
- This paper consists of TWO Sections; Section A and Section B.
- Answer ALL the questions in both Section A and B in the spaces provided.
- ALL working MUST be clearly shown.
- Candidates should check the question paper to ascertain that all the 8 pages are printed as indicated and that no questions are missing.
- Candidates should answer the questions in English.
Where necessary, take:
g = 10N/kg
Density of water = 1000kg/m3
Section A: 25 marks
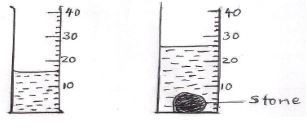
- The figure below shows the volume of water in a measuring cylinder before and after immersing a stone. If the mass of the stone is 125g, determine its density. (3mks)
- A drug manufacturer gives the mass of an active ingredient in a table as 4.0mg. Express this quantity in kilogrammes (1mk)
- Giving an example, define the termderived quantities. (2mks)
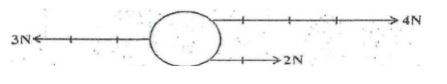
- A body is acted upon by three forces as shown below.
Draw on the body below to show the resultant force acting on it. (2mks) - Stateany two the difference between mass and weight (2mks)
- Explain why water wets the glass while mercury does not.(2mks)
- Name the instruments you would use to measure each of the following:
- The length of a football field. (1mk)
- The height of a 20 litrejerrican (1mk)
- The circumference of your waist. (1mk)
- The water level in a burette is 30cm3. If 55 drops of water fall from the burette and the average volume of one drop is 0.12cm3, what is the final water level in the burette? (2mks)
- A man has a mass of 70kg. Determine
- His weight on earth, where the gravitational field strength is 10N/kg. (2mks)
- The gravitational field strength on the moon if his weight on the moon is 119N (2mks)
- With the aid of a diagram show that pressure increases with depth (2mk)
- Describe an experiment to show that matter is made up of small particles (2mk)
Section B: 55 marks
-
- Define pressure and state its SI unit (2mks)
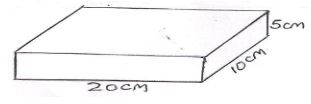
- The figure below shows the measurements of a solid of mass 50kg.
Determine:- The weight of the solid (1mk)
- The minimum pressure the solid can exert on a flat surface (3mks)
- The maximum pressure the solid can exert on a flat surface (3mks)
- A sea diver is 35m below the surface of sea water. If the density of the sea water is 1.03gcm-3, the atmospheric pressure 103 000Nm-2 and g is 10N/kg; determine the total pressure on him. (3mks)
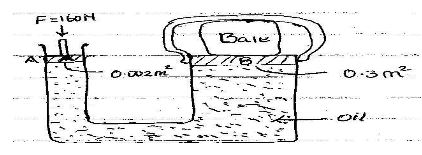
- The diagram below shows a simple hydraulic lift
If aforce of 160N is applied on the small piston. Determine:- The pressure at the side of small piston A. (2mks)
- Pressure experienced by the oil (1mk)
- Force produced on Large pistonB to compress the bale (3mks)
- State two factors that affect pressure other than depth. (2mk)
-
- Define the term diffusion (1mk)
- Distinguish between solid and liquid states of matter in terms of intermolecular forces. (2mks)
- Hydrochloric gas diffuses faster than ammonia gas. Suggest a reason for this observation(2mks)
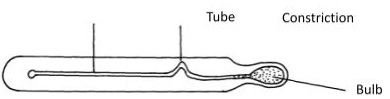
- Brown motion of smoke particles can be studied by using the apparatus shown below. To observe the motion, some smoke is enclosed in the smoke cell and then observed through the microscope.
- Explain the role of the lamp, lens and microscope in the experiment (3mks)
- State and explain the nature of the observed motion of the smoke particles(2 marks)
- State what will be observed about the motion of the smoke particles if the temperature surrounding the smoke cell is slightly raised. (1 mark)
- The figure below shows a beaker filled with water. Some potassium permanganate was gently introduced at the bottom of the beaker at the position shown.Figure (ii) shows uniform purple colour of potassium permanganate solution after about 10 minutes
Explain how the appearance in figure(ii) was caused. (2mks) -
- Define the term temperature and state its SI units (2mks)
- The figure is of a below represent mercury in a clinical thermometer.
Explain why;- There is a constriction on the tube (1mk)
- The bulb is thin (1mk)
- Mercury is the mostly preferred thermometric liquid in clinical thermometer than alcohol (2mks)
- A clinical thermometer is likely to break if sterilized using boiling water (1mk)
- Convert
- emperatures of -173°C to Kelvin (2mks)
- Temperatures of 376K to °C. (2mks)
-
- Giving an example in each, state the difference between scalar and vector quantity.(4marks)
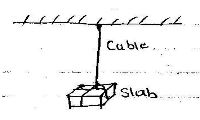
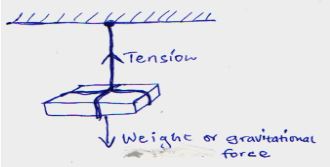
- A concrete slab of mass 20g is held by a steel cable of a crane as shown below. Name and show the forces acting on the slab (2mks)
- State any two examples of contact forces. (2mks)
- State any threeeffects of a force on a body (3mks)
MARKING SCHEME
Section A: 25 marks
- The figure below shows the volume of water in a measuring cylinder before and after immersing a stone. If the mass of the stone is 125g, determine its density. (3mks)
V=26-16 = 10cm3
Density= mass/v = 125/10 = 12.5g/cm3 or 12500kg/m3 - A drug manufacturer gives the mass of an active ingredient in a table as 4.0mg. Express this quantity in kilogrammes (1mk)
4/1.0 x10⁶= 4.0 x 10-6kg - Giving an example, define the termderived quantities. (2mks)
Quantities which we choose to define interms of other quantities.Examples area and volume - A body is acted upon by three forces as shown below.
Draw on the body below to show the resultant force acting on it. (2mks) - Stateany two the difference between mass and weight (2mks)
- Mass is quantity of matter contained in a substance while weight is pull of gravity.
- SI unit of mass is kilogramme while that of weight is Newton
- Explain why water wets the glass while mercury does not.(2mks)
- Water wets the glass because adhesive force between water and glass molecules is greater than cohesive force between water molecules. Mercury does not wet the glass because cohesive force between mercury molecules is greater than adhesive force between glass molecules and mercury molecules .
- Water wets the glass because adhesive force between water and glass molecules is greater than cohesive force between water molecules. Mercury does not wet the glass because cohesive force between mercury molecules is greater than adhesive force between glass molecules and mercury molecules .
- Name the instruments you would use to measure each of the following:
- The length of a football field. (1mk)
- Surveyor’s tape measure
- Surveyor’s tape measure
- The height of a 20 litrejerrican (1mk)
- Carpenterstape measure/metre rule
- Carpenterstape measure/metre rule
- The circumference of your waist. (1mk)
- Tailors tape measure
- Tailors tape measure
- The length of a football field. (1mk)
- The water level in a burette is 30cm3. If 55 drops of water fall from the burette and the average volume of one drop is 0.12cm3, what is the final water level in the burette? (2mks)
- 0.12x 5.5 =6.6cm3
Final burette readings 30.0+ 6.6 = 36.6 cm3
- 0.12x 5.5 =6.6cm3
- A man has a mass of 70kg. Determine
- His weight on earth, where the gravitational field strength is 10N/kg. (2mks)
- W= mg =70x10
= 700N
- W= mg =70x10
- The gravitational field strength on the moon if his weight on the moon is 119N (2mks)
- Gravitational field strength= w/m
= 119/70= 1.7N/kg
- Gravitational field strength= w/m
- His weight on earth, where the gravitational field strength is 10N/kg. (2mks)
- With the aid of a diagram show that pressure increases with depth (2mk)
- Describe an experiment to show that matter is made up of small particles (2mk)
- Tear a small piece of paper from your book, cut it into small pieces. Cut the small pieces into other further small pieces.
- Continue until the papers obtained cannot be further cut in smaller pieces. The pieces of the paper obtained at the end illustrate constituents of matter.
- Dissolving a solute in a solvent.
- Grinding a piece of chalk.
- Diluting potassium manganate IV and copper II sulphate
Section B: 55 marks
-
- Define pressure and state its SI unit (2mks)
- Pressure is force acting normally per unit area SI unit: Newton per square meter or pascal
- Pressure is force acting normally per unit area SI unit: Newton per square meter or pascal
- The figure below shows the measurements of a solid of mass 50kg.
Determine:- The weight of the solid (1mk)
- W=mg = 50 x 10 = 500N
- W=mg = 50 x 10 = 500N
- The minimum pressure the solid can exert on a flat surface (3mks)
- Pmin = force/Max Area =500/0.02= 25000N/m2
- Pmin = force/Max Area =500/0.02= 25000N/m2
- The maximum pressure the solid can exert on a flat surface (3mks)
- Pmax = force/Min Area = 500/0.005 = 100000N/m2
- Pmax = force/Min Area = 500/0.005 = 100000N/m2
- A sea diver is 35m below the surface of sea water. If the density of the sea water is 1.03gcm-3, the atmospheric pressure 103 000Nm-2 and g is 10N/kg; determine the total pressure on him. (3mks)
- Total pressure= pressure due to water+ atmospheric pressure
= 1030 x 35 x 10 + 103000
= 360500+ 103000
= 463500 Pascals
- Total pressure= pressure due to water+ atmospheric pressure
- The weight of the solid (1mk)
- Define pressure and state its SI unit (2mks)
- The diagram below shows a simple hydraulic lift
If aforce of 160N is applied on the small piston. Determine:- The pressure at the side of small piston A. (2mks)
- Pressure at A =F/A =160/0.002 = 80000N/m2
- Pressure at A =F/A =160/0.002 = 80000N/m2
- Pressure experienced by the oil (1mk)
- 80000N/m2
- 80000N/m2
- Force produced on Large pistonB to compress the bale (3mks)
- Force at B = Pressure x Area at B =80000x 0.3 =24000N/m2
- Force at B = Pressure x Area at B =80000x 0.3 =24000N/m2
- State two factors that affect pressure other than depth. (2mk)
- Density and gravitational field strength,
- Density and gravitational field strength,
- The pressure at the side of small piston A. (2mks)
-
- Define the term diffusion (1mk)
- Movement of molecules from where they’re highly concentrated to where they are lowly concentrated
- Movement of molecules from where they’re highly concentrated to where they are lowly concentrated
- Distinguish between solid and liquid states of matter in terms of intermolecular forces. (2mks)
- Solid molecules have less intermolecular distances between theirmoleculesthus stronger intermolecular forces than liquids; Liquid molecules have relatively greater intermolecular distancesthus weaker intermolecular forces than solid molecules.
- Solid molecules have less intermolecular distances between theirmoleculesthus stronger intermolecular forces than liquids; Liquid molecules have relatively greater intermolecular distancesthus weaker intermolecular forces than solid molecules.
- Hydrochloric gas diffuses faster than ammonia gas. Suggest a reason for this observation(2mks)
- Diffusion is affected by density of a molecule. Ammonia gas which has a less density than HCL gas diffuses faster than HCL gas which has a greater density.
- Diffusion is affected by density of a molecule. Ammonia gas which has a less density than HCL gas diffuses faster than HCL gas which has a greater density.
- Define the term diffusion (1mk)
- Brown motion of smoke particles can be studied by using the apparatus shown below. To observe the motion, some smoke is enclosed in the smoke cell and then observed through the microscope.
- Explain the role of the lamp, lens and microscope in the experiment (3mks)
- Lens-concentrate/focus light to the smoke cell
- Lamp – illuminates/provides light to the smoke cell
- Microscope-magnify/enlarge the smoke particles
- State and explain the nature of the observed motion of the smoke particles(2 marks)
- Random or Brownian motion: due to collision with the invisible air molecules
- Random or Brownian motion: due to collision with the invisible air molecules
- State what will be observed about the motion of the smoke particles if the temperature surrounding the smoke cell is slightly raised. (1 mark)
- Smoke particles move faster than before or increase their speed of their movement
- Smoke particles move faster than before or increase their speed of their movement
- Explain the role of the lamp, lens and microscope in the experiment (3mks)
- The figure below shows a beaker filled with water. Some potassium permanganate was gently introduced at the bottom of the beaker at the position shown.Figure (ii) shows uniform purple colour of potassium permanganate solution after about 10 minutes
Explain how the appearance in figure(ii) was caused. (2mks)- diffussion
- Potassium permangate move from a region of high concentration to region of low concentration until a uniform purple colour is obtained at end of the experiment
-
- Define the term temperature and state its SI units (2mks)
- Degree of hotness or coldness of a body or place. SI unit;Kelvin
- Degree of hotness or coldness of a body or place. SI unit;Kelvin
- The figure is of a below represent mercury in a clinical thermometer.
Explain why;- There is a constriction on the tube (1mk)
- Cut the mercury thread hence allow/enable temperature readings to be taken before mercury flows back to the bulb
- Cut the mercury thread hence allow/enable temperature readings to be taken before mercury flows back to the bulb
- The bulb is thin (1mk)
- Increase sensitivity of the thermometer
- Increase sensitivity of the thermometer
- Mercury is the mostly preferred thermometric liquid in clinical thermometer than alcohol (2mks)
- Mercury has relatively uniform expansion and contraction as compared to alcohol.
- Mercury is more visible as compared to alcohol which is less visible.
- A clinical thermometer is likely to break if sterilized using boiling water (1mk)
- Because it measures a maximum of 430c while boiling water is at 1000c thus breaking it.
- Because it measures a maximum of 430c while boiling water is at 1000c thus breaking it.
- There is a constriction on the tube (1mk)
- Convert
- Temperatures of -173°C to Kelvin (2mks)
- -173+273 = 100K
- -173+273 = 100K
- Temperatures of 376K to °C. (2mks)
- 376-273 = 1030c
- Temperatures of -173°C to Kelvin (2mks)
- Define the term temperature and state its SI units (2mks)
-
- Giving an example in each, state the difference between scalar and vector quantity.(4marks)
- Scalar quantity have size/magnitude only e.g. mass and density
- Vector quantity have both magnitude and direction e.g. momentum, weight and velocity.
- Scalar quantity have size/magnitude only e.g. mass and density
- A concrete slab of mass 20g is held by a steel cable of a crane as shown below. Name and show the forces acting on the slab (2mks)
- State any two examples of contact forces. (2mks)
- Frictional force, action and reaction force, cohesive and adhesive forces.
- Frictional force, action and reaction force, cohesive and adhesive forces.
- State any threeeffects of a force on a body (3mks)
- makes a stationary body to move
- stops a moving body
- distorts/deforms a moving body
- changes direction of a moving body
- Giving an example in each, state the difference between scalar and vector quantity.(4marks)
Download Physics Questions and Answers - Form 1 Opener Exam Term 3 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students