QUESTIONS
Answer all the questions in the spaces provided.
- State any two branches of Chemistry. (2 mks)
-
- What is a drug? (1 mk)
- List any three frequently abused drugs? (3 mks)
- Outline four roles of Chemistry in the society. (4 mks)
- Identify the following apparatus as they are used and found in a Chemistry Laboratory.(4 mks)
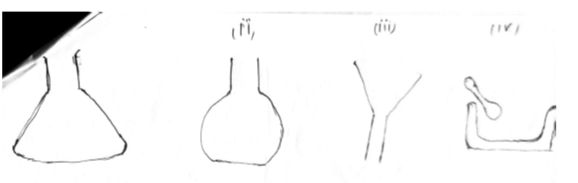
-
- Define a flame as used in Chemistry . (1 mk)
- State the differences between luminous and non-luminous flames. (4 mks)
Luminous
Non-Luminous
-
- Identify the following apparatus and state its function as used in the laboratory.(2 mks)
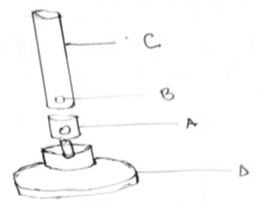
- State the uses of the labeled parts; A, B, C and D. (4 mks)
A –
B –
C -
D -
- Identify the following apparatus and state its function as used in the laboratory.(2 mks)
-
- State the three states of matter. (1 ½ mks)
- Identify the following processes that show the changes of state that occur when a substance is heated and then cooled. (6 mks)
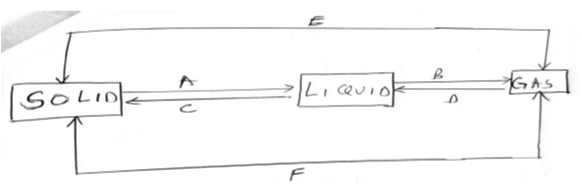
-
- What is a mixture? (1 ½ mks)
- State any four physical methods of separating mixtures. (2 mks)
-
- Define the following terms:- (3 mks)
- Atom –
- Compound –
- An element –
- State the differences between temporary and permanent changes. (4 mks)
Temporary
Permanent
- Define the following terms:- (3 mks)
- List any six laboratory rules and regulations of a Chemistry Laboratory. (6 mks)
- Name any four career opportunities of Chemistry as a subject. (4 mks)
- State why most of laboratory apparatus are made of glasses. (3 mks)
- State three long-term effects of drug-abuse. (3 mks)
- The following diagrams represent a non-luminous flame of a Bunsen burner.
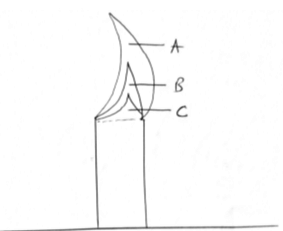
- Name the parts of the flame labeled A, B and C. (1 ½ mks)
- Which part is the hottest? ( ½ mks)
- A non-luminous flame is preferred for heating. Explain. (2 mks)
-
- Name the other type of flame produced by a Bunsen burner. (1 mk)
- Under what conditions does the Bunsen burner produce the flame you have named in d(i) above? (1 mk)
- State the effect of impurities on melting on boiling points. (1 mk)
-
- Name the elements present in the following compounds:
- Calcium carbonate –
- Magnesium nitride -
- Write a word equation for the reaction between:-
- Carbon and Oxygen.
- Sodium and oxygen.
- Name the elements present in the following compounds:
MARKING SCHEME
Answer all the questions in the spaces provided.
- State any two branches of Chemistry. (2 mks)
physical chemistry
inorganic chemistry -
- What is a drug? (1 mk)
A substance that alters the normal functioning of the body when taken - List any three frequently abused drugs? (3 mks)
tobacco
cocaine
bhang
- What is a drug? (1 mk)
- Outline four roles of Chemistry in the society. (4 mks)
manufacture of drugs
as a career subject
food production
manufacture of detergents - Identify the following apparatus as they are used and found in a Chemistry Laboratory.(4 mks)

- conical flask
- flat bottomed flask
- filter funnel
- motar and pestle
-
- Define a flame as used in Chemistry . (1 mk)
is a zone of burning gases - State the differences between luminous and non-luminous flames. (4 mks)
Luminous
Non-Luminous
has four zones three zones large and steady small and steady emits soot non-sooty quiet noisy not so hot very hot
- Define a flame as used in Chemistry . (1 mk)
-
- Identify the following apparatus and state its function as used in the laboratory.(2 mks)

bunsen burner - used for heating substances during experiments - State the uses of the labeled parts; A, B, C and D. (4 mks)
collar - regulates the amount of air that gets into the chimney
air hole - allows air to enter the chimney and mix with laboratory gas
chimney - space where the laboratory gas mixes with air
base - supports other parts of the bunsen burner
- Identify the following apparatus and state its function as used in the laboratory.(2 mks)
-
- State the three states of matter. (1 ½ mks)
solid
liquid
gases - Identify the following processes that show the changes of state that occur when a substance is heated and then cooled. (6 mks)

A - melting
B - vaporisation
C - freezing
D - condensation
E - sublimation
F - deposition
- State the three states of matter. (1 ½ mks)
-
- What is a mixture? (1 ½ mks)
consists of two or more substances combined together that one may use physical means to separate - State any four physical methods of separating mixtures. (2 mks)
distillation
filtering
use of magnets
winnowing
- What is a mixture? (1 ½ mks)
-
- Define the following terms:- (3 mks)
- Atom – the smallest particle of an element that can take part in a chemical change
- Compound – a pure substance that is made up of two or more elements that are chemically combined
- An element – a pure substance that can not be split into simpler substances by chemical means
- State the differences between temporary and permanent changes. (4 mks)
Temporary
Permanent
reversible irreversible no new substance formed new substance is formed no change in mass change in mass
- Define the following terms:- (3 mks)
- List any six laboratory rules and regulations of a Chemistry Laboratory. (6 mks)
never taste or eat
never run around in the laboratory
never smell the gases directly
always extinguish flames that are not in use
always use a claen spatula for scooping substances
always consult your teacher - Name any four career opportunities of Chemistry as a subject. (4 mks)
chemistry teacher
pharmacy
engineer
a doctor - State why most of laboratory apparatus are made of glasses. (3 mks)
not react with most of the substances
easy to clean
transparent - State three long-term effects of drug-abuse. (3 mks)
stress
depression
hallucination - The following diagrams represent a non-luminous flame of a Bunsen burner.

- Name the parts of the flame labeled A, B and C. (1 ½ mks)
A - pale blue flame
B - green blue zone
C - almost colourless - Which part is the hottest? ( ½ mks) A
- A non-luminous flame is preferred for heating. Explain. (2 mks)
produces alot of heat compared to that of luminous flame -
- Name the other type of flame produced by a Bunsen burner. (1 mk)
luminous - Under what conditions does the Bunsen burner produce the flame you have named in d(i) above? (1 mk)
when the air hole is partially opened or closed
- Name the other type of flame produced by a Bunsen burner. (1 mk)
- Name the parts of the flame labeled A, B and C. (1 ½ mks)
- State the effect of impurities on melting on boiling points. (1 mk)
lowers the melting point and rises the boiling point -
- Name the elements present in the following compounds:
- Calcium carbonate – calcium, carbon and oxygen
- Magnesium nitride - magnesium and nitrogen
- Write a word equation for the reaction between:-
- Carbon and Oxygen.
carbon + oxygen → carbon(IV) oxide - Sodium and oxygen.
Sodium + Oxygen → Sodium oxide
- Carbon and Oxygen.
- Name the elements present in the following compounds:
Download Chemistry Questions and Answers - Form 1 End Term 1 Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

