- Define the terms below
- Atomic number (1mk)
- Mass number (1mk)
- An atom can be represented as 126W. What do the numbers 12 and 6 represent? (2mk)
- Differentiate between corrosion and rusting (2mk)
- Describe how liquid air can be obtained (3mk)
- Complete and balance the following equations
- H2(g) + O2(g )-----------------
- NaOH (aq)+ HCL(aq) ------------------------
- Name the radicals below
- PO43- (1mk)
- SO42- (1mk)
- OH- (1mk)
- The grid below is part of the periodic table , the letters are not actual symbol
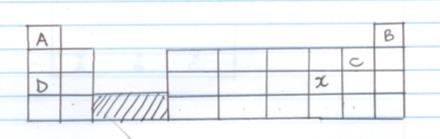
Study it and answer the questions below- What do the rows and columns represent?
- Write the electronic arrangement of element C (1mk)
- Draw the atomic structure of C (2mk)
- On the grid show the position of the element with the following electronic arrangement
E=2.1 F=2.8.5 (2mk)
-
- Carbon atom can be represented as 146C and 126C State the name given to such atoms (1mk)
- Calculate the relative atomic mass of carbon, if atom 146C has relative abundance of 1.1% (3mk)
- The set up below was used to separate liquid A (boiling point of 78oC) and liquid B (boiling point 100oC) mixture
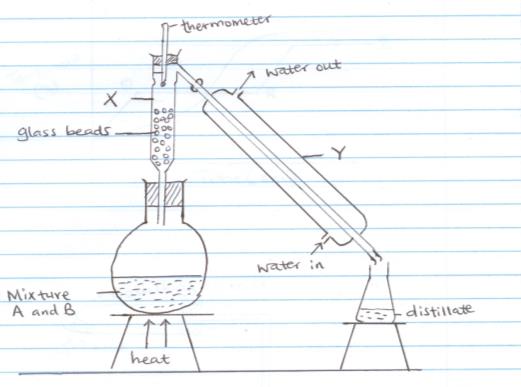
- Name the process shown above (1mk)
- Name the apparatus labelled
X (1mk)
Y (1mk) - Which liquid was collected first as the distillate? Give a reason for your answer. (2mk)
- State the role of glass beads. (1mk)
- State two reasons why non luminous flame is used in the laboratory instead of the luminous flame. (2mk)
- The diagram below shows chromatograms for substances A, B and C
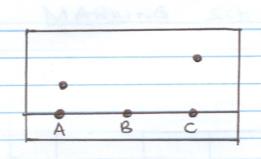
- Name the technique used to separate the substances above. (1mk
- Show the solvent front on the diagram above. (1mk)
- What does the chromatogram indicate about
- Substance A (1mk)
- Substance B (1mk)
- Substance D contains substances A and C. Show the chromatogram of D on the diagram (2mk)
- Study the following table below and answer the questions that follows ( The letters do not represent the actual symbols of the element)
Atom Atomic number Mass Number M 9 19 N 12 24 O 13 26 P 13 27 Q 19 39 - What is the electronic configuration of Ion of M and P (2mk)
- Identify the non-metallic element. Give a reason (2mk)
- To which period do the following elements belong?
- M
- P
- Q
- Giving a reason state the group to which element M belong. (2mk)
- Write the formulae of the most stable ions of M and Q (2mk)
- Write the formula of the compound formed between element M and Q (1mk)
- Give the number of neutrons in the following atoms (2mk)
N
O
- The curves below represents variations of temperature with time when samples of solids were heated separately
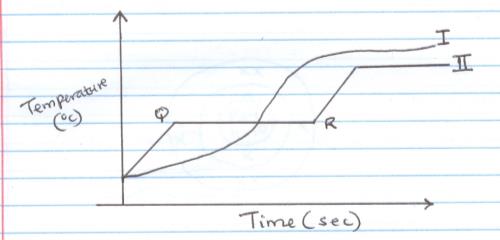
- Which curve shows the variation of temperature for a pure solid? Explain (2mk)
- Explain why the temperature does not rise between QR. (1mk)
- Name two apparatus used to measure fixed accurate volume of liquids in the laboratory (2mk)
- Name the most suitable method used to obtain the following substances from their mixture (2mk)
- Oil from nuts
- Ammonium Chloride from sodium chloride
- Salt from salty water
- Iron filings from sand
- Element B and C belong to the second group of the periodic table. Element B is above C in the group
- How does their atomic radii compare? Explain? (2mk)
- How does reactivity of these elements with dilute acids compare. Explain(2mk)
- Write an equation for the reaction between element C with dilute hydrochloric acid (1mk)
- Name two elements which belong to group II and state their uses.(2mk)
- The atom of element L has electronic configuration of 2.8.6
- What is the valency of L (1mk)
- Draw the structure of the ion formed by L (2mk
- Write the formulae of the compound formed between element L and hydrogen (1mk)
- State two industrial uses of oxygen gas (2mk)
- Fill in the colour of the following indicators in audit and basic solution (2mk)
Name of indicator Acidic Basic Phenopthalin Methyl orange
- Study the set up below and answer the questions that follow
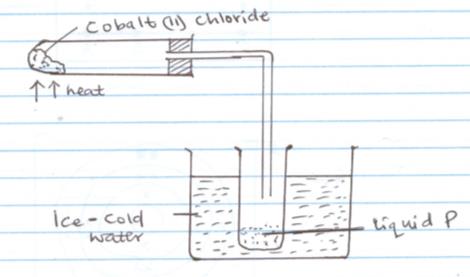
- What is the colour of hydrated cobalt (II) Chloride (1mk)
- What is the use of ice cold water (1mk)
- How can liquid P be confirmed to be water (1mk)
- Write the chemical formulae of the following compounds
- Lead(II) carbonate (1mk)
- Magnesium nitrate (1mk)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY - Form 2 End of Term 1 2019 Examinations.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

