-
- Atomic number is the number of protons in the nucleus of an atom.
- Mass number is the sum of the protons and neutrons in the nucleus of an atom of an element.
- 6- atomic number, 12- Atomic mass / mass number.
- Corrosion occurs only on metals while rusting occurs on irons only
- When the air expands its temperature fall and it is passed back to cool fresh gases leaving the compressor. It is then itself returned to the compressor as the cycle is repeated several times the temperature falling each time until eventually liquefaction occurs.
- water
- 2H2+02= 2H2O
- NaOH(aq) +HCI (aq)= NaCl(aq)+H2O(l)
-
- Phosphate
- Sulphate
- Hydroxide. 1 mk
-
-
- Rows-periods
- Columns-group
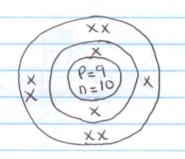
- 2.7
-

- E 2.1 F 2.8.1
E------- group 1, period 2
V------ group (v), period 3.
-
-
- Isotopes.
- 12C (100−1.1) = (98−9)
RAM = 12 x 98.9/100 + 14 x 1.1/100
= 11.86+0.154
=12.022
-
- Fractional distillation
-
- X- Fractionating column
- Y- Liebig Condenser
- Liquid A, It has a lower boiling point
- To increase the surface area for the condenser.
-
- Is hotter than luminous flame
- Does not dirtify the apparatus with soot
-
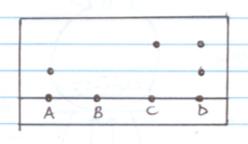
- Chromatography
- On the diagraM
-
- A- is a pure substance
- B- is insoluble in the solvent used.
- Diagram
-
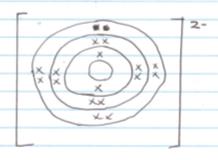
- M- ---------- 2.8 1mk
P3+ ---------2.8 - M (1mk) reacts by gaining(1mk) an electron to attain stability.
- M- Period 2 1 mk
P- Period 3 1 mk
Q-Period 4 1 mk - group 7 (1mk) - It has seven (1mk) valence electrons
- M-
Q+ - MQ (1mk) or KF (reject QM)
- N n=24-12=12 1 mk
O n= 26-13 = 13 1 mk
- M- ---------- 2.8 1mk
-
- Curve II
- It has sharp melting and boiling points
- The heat supplied (latent heat of fusion) helps to overcome the forces of attraction between the solid particles.
- Curve II
-
- Pipette 1 mk
- Volumetric flask 1 mk
-
- Solvent extraction ½ mk each
- Sublimation
- Simple distillation (reject distillation or fractional distillation
- Using a magnet.
-
- Atomic radii increase down the group due to increase in energy level. 1mk
- C reacts more vigorously than B
C has larger atomic radius than B thus looses electrons readily - C (g) +2HCL (aq) __________ C CL2 (aq) + H2 (g)
- Mg ½ & Ca their uses. (each use ½ mk)
-
- Valency 2
- H2L
-
- Used in respiratory aid for patients with breathing difficulties. 1mk
- Oxygen is used as ex ethyne flame for welding. 1 mk
- Liquid oxygen is used to burn the fuel in space rockets. ½ mk each
-
Name of Indicator Acidic Basic Phenopthalin Colourless Pink Methyl Orange Pink Yellow
-
- pink 1 mk
- to condense the vapour forming liquid P 1 mk
- Using anhydrous copper (II) (1mk) sulphate turns from white to blue 1mk
Or
Using anhydrous ( ½ mk) cobalt (II) chloride turns from blue to pink ½ mk
Or
Determining its (½ mk) boiling point. Its 1000c ½ mk
-
- PbCO3 1 mk
- Mg(NO3)2 1 mk
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY Marking Scheme - Form 2 End of Term 1 2019 Examinations.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students