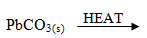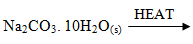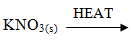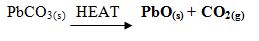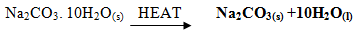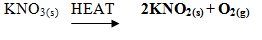Instructions to students
- Answer all the questions in the spaces provided.
- KNEC mathematical tables and silent non-programmable electronic calculators may be used for calculations.
- All working MUST be clearly shown where necessary.
- Students should answer the questions in English.

QUESTIONS
- Name a method that can be used to separate each of the following substances. (3mks)
- A mixture of petrol and diesel.
- Kerosene and water.
- Food coloring ingredients in a sauce.
- The table below shoes the formulae of elements P, Q, R and S (not actual symbols) and their chlorides.
Elements P Q R S Formulae of chlorides PCL QCL2 RCL3 SCL5 - State the group in which element Q belongs. (1mrk)
- Identify one element which is a non-metal. (1mk)
- Write down the formulae of P oxide. (1mk)
- Hydrogen can be prepared by passing steam over heated Zinc powder as shown in the diagram
- Write down the chemical reaction that produces hydrogen gas. (1mrk)
- Explain why hydrogen should be burned if not collected over water. (1mrk)
- Give another metal that can be used instead of Zinc. (1mrk)
- A piece of sodium metal was placed in a trough half filled with cold water. State the observations that were made. (3mrks)
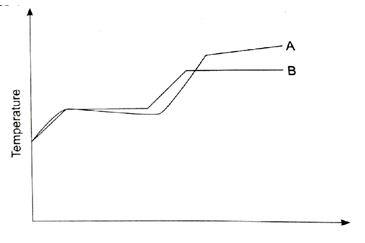
- The curves below represents the variation of temperature with time when pure and impure samples of a solid were heated separately.
- Which curve shows the variation in temperature of the pure solid. Explain (2Mrks)
- State the effect of impurities in the melting and boiling points of a pure substance. (2Mrks)
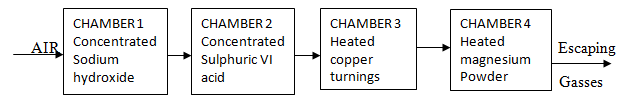
- Air was passed through several reagents as shown below;
- Name the main inactive component of air (1mk)
- Name the components of air that are removed in the following chambers
- Chamber 1
- Chamber 3
- Chamber 4
- What is the purpose of passing air through concentrated Sulphuric (VI) acid? (1mk)
- Write a chemical equation for the reaction which takes place in
- Chamber 1
- Chamber 4
- Explain the observation made in chamber 3 during the reaction. (2mrks)
- Name one gas which escapes from the scheme above. (1mrk)
-
- Distinguish between hygroscopy and efflorescence. (2mrks)
- Starting with lead (II) oxide describe how you would prepare Lead (II) sulphate (3mrks)
-
- discuss the criteria for testing purity of water. (2mrks)
- write the word equations for the reaction between dilute hydrochloric acid and the following.
- magnesium oxide
- calcium hydrogen carbonate
- zinc metal
- potassium hydroxide (4mrks)
-
- Using dots and crosses to represent electrons, draw a diagram to show bonding in Sodium Chloride(NaCl) (2mrks)
- name and draw two apparatus used in measuring exact volumes of solutions in the laboratory (2mrks)
- Both ions Y2- and Z2+ have an electron configuration of 2.8.8
- Write the electron arrangement for (2mrks)
Y
Z - What is the mass number of atom Z given that it has 20 neutrons (1mrk)
- Write the electron arrangement for (2mrks)
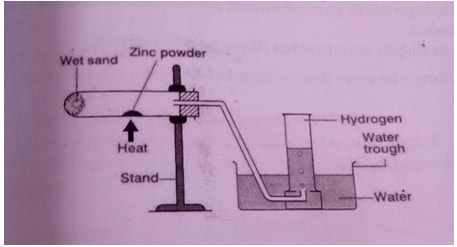
- The diagram on the next page shows a set up which was used by a student to investigate the effect of electricity on molten Lead (II) Bromide.
- Explain the observation at the cathode (2mrks)
- Why does solid lead (II) Bromide not allow the passage of electricity (2mrks)
- Write equations to show the reactions taking place
- At the cathode (1mrk)
- At the anode (1mrk)
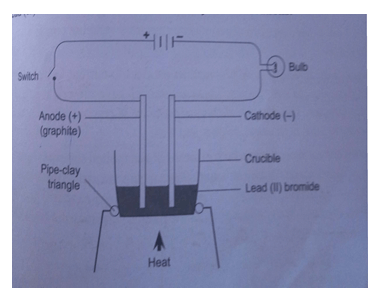
- Study the set up below and answer the questions that follow
- Identify gas X (1mrk)
- Write a chemical equation for the reaction liberating gas X (1mrk)
- Why is it not advisable to use calcium in this method of preparing gas X? (2mrks)
- Give the use of anhydrous calcium chloride in the U-tube (1mrk)
- Name another substance that could serve the same purpose as anhydrous calcium chloride (1mrk)
- Name the method used to collect gas X (1mrk)
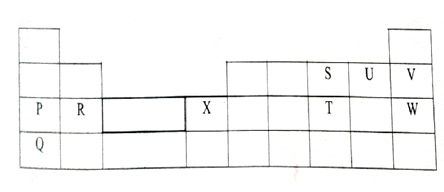
- The grid below shows part of the periodic table. Use it to answer the questions that follow.
- Which of the elements has the largest atomic radius? Explain (2mrks)
- Identify the most reactive metal. Explain (2mrks)
- Name the chemical family to which P and Q belong. (1mrk)
- Compare the atomic radius of S and U. Explain (2mrks)
- Select an element that does not form an ion. Explain (2mrks)
- Give the formula of one stable cation with an electron arrangement of 2.8.8 (1mrk)
-
- Define the term isotope (1mrk)
- Chlorine gas has a relative atomic mass of 35.5. It is made up of two isotopes 3517CL and 3717CL. Determine the relative abundance of each isotope in the chlorine gas. (3mrks)
- Write a balanced equation for the decomposition of the following solids (3mrks)
-
- Though Sodium and aluminium are in the same period and are both metals, aluminium is a better conductor of electricity. Explain (2mrks)
-
- List any three uses of oxygen gas` (3mrks)
- State the conditions necessary for rusting. (2Mrks)

MARKING SCHEME
- Name a method that can be used to separate each of the following substances. (3mks)
- A mixture of petrol and diesel
- Fractional distillation
- Kerosene and water.
- Use of a separating funnel
- Food coloring ingredients in a sauce.
- Chromatography
- A mixture of petrol and diesel
- The table below shoes the formulae of elements P, Q, R and S (not actual symbols) and their chlorides.
- State the group in which element Q belongs. (1mrk)
- Group II
- Identify one element which is a non-metal. (1mk)
- Element S
- Write down the formulae of P oxide. (1mk)
- P2O
- State the group in which element Q belongs. (1mrk)
- Hydrogen can be prepared by passing steam over heated Zinc powder as shown in the diagram below
- Write down the chemical reaction that produces hydrogen gas. (1mrk)
- Zn(s)+ H2O(g) ZnO(s)+H2(g)
- Explain why hydrogen should be burned if not collected over water. (1mrk)
- A mixture of hydrogen and gas explodes.
- Give another metal that can be used instead of Zinc. (1mrk)
- Magnesium, Iron, Lead or Copper
- Write down the chemical reaction that produces hydrogen gas. (1mrk)
- A piece of sodium metal was placed in a trough half filled with cold water. State the observations that were made. (3mrks)
- The metal darts around the water surface.
- The metal melts into a silvery ball.
- There is production of a hissing sound.
- The curves below represents the variation of temperature with time when pure and impire samples of a solid were heated separately.
- Which curve shows the variation in temperature of the pure solid. Explain (2Mrks)
- Constant melting points and boiling points
- State the effect of impurities in the melting and boiling points of a pure substance. (2Mrks)
- Melting point – Lower the melting point.
- Boiling point – Raises the boiling point.
- Which curve shows the variation in temperature of the pure solid. Explain (2Mrks)
- Air was passed through several reagents as shown below;
- Name the main inactive component of air (1mk)
- Nitrogen gas
- Name the components of air that are removed in the following chambers
- Chamber 1
- CO2 gas
- Chamber 3
- O2 gas
- Chamber 4
- N2 gas
- Chamber 1
- What is the purpose of passing air through concentrated Sulphuric VI acid? (1mk)
- To remove /absorb water vapor/drying agent
- Write a chemical equation for the reaction which takes place in
- Chamber 1
- 2NaOH(aq) + CO2(g) → Na2CO3(s) + H2O(l) penalize ½ if state symbols are missing/wrong
- Chamber 4
- 3Mg(s) + N2(g) → Mg3N2(s) penalize fully if not balanced
- Chamber 1
- Explain the observation made in chamber 3 during the reaction. (2mrks)
- Brown solid changes to black
- Brown copper metal oxidised form copper II oxide
- Name one gas which escapes from the scheme above. (1mrk)
- Argon
- Neon
- Helium
- Name the main inactive component of air (1mk)
- Distinguish between hygroscopy and efflorescence. (2mrks)
- Hygroscopy is a process which salts exposed to the atmosphere become dump.
- Efflorescence is a process by which salts lose water of crystallization to the atmosphere.
- Starting with lead II oxide describe how you would prepare Lead II sulphate (3mrks)
- To a given volume of nitric acid, add excess Lead II oxide until some residue is left in the beaker.
- Filter to obtain Lead II nitrate solution and Lead II oxide residue.
- To the filtrate add excess solution of Na2SO4 to ensure complete precipitation.
- Filter to obtain PbSO4 as residue and NaNo3 solution as filtrate.
- Rinse the residue and dry between filter papers.
- Distinguish between hygroscopy and efflorescence. (2mrks)
- Describe a chemical test to differentiate between carbon IV oxide and carbon II oxide gas. (2mrks)
- Pass the two gasses separately through Ca(OH)2 solution .White precipitate is observed with Carbon IV oxide while no white ppt is formed with carbon II oxide
- Give 3 uses of carbon IV oxide gas. (3mrks)
- As a refrigerating agent for perishable goods.
- Used as a fire extinguisher.
- Used in manufacture of sodium carbonate in Solvay process.
- Describe a chemical test to differentiate between carbon IV oxide and carbon II oxide gas. (2mrks)
- Using dots and crosses to represent electrons, draw a diagram to show bonding in Sodium Chloride(Nacl) (2mrks)
- Both graphite and diamond are allotropes of carbon. Graphite conducts electricity whereas diamond does not. Explain (2mrks)
- The presence of delocalized electrons in the structure of graphite explains its electrical conductivity. Diamond has no delocalized electrons in its structure.
- Both ions Y2- and Z2+ have an electron configuration of 2.8.8
- Write the electron arrangement for (2mrks)
- Y 2.8.6
- Z 2.8.8.2
- What is the mass number of atom Z given that it has 20 neutrons (1mrk)
- 40 Protons + neutrons = Mass no
- 20+20 = 40
- Write the electron arrangement for (2mrks)
- The diagram on the next page shows a set up which was used by a student to investigate the effect of electricity on molten Lead II Bromide.
- Explain the observation at the cathode (2mrks)
- Grey deposits of lead beads are deposited at the cathode
- Why does solid lead II Bromide not allow the passage of electricity (2mrks)
- Lead II Bromide solid is a molecular substance and does not contain ions which are responsible for electrical conductivity.
- Write equations to show the reactions taking place
- At the cathode (1mrk)
- Pb2+(aq) +2e- → Pb(s)
- At the anode (1mrk)
- 2Br–(aq) → Br2(l)+ 2e-
- At the cathode (1mrk)
- Explain the observation at the cathode (2mrks)
- Study the set up in the next page and answer the questions that follow
- Identify gas X (1mrk)
- Hydrogen gas
- Write a chemical equation for the reaction liberating gas X (1mrk)
- Zn(s)+ 2HCl(aq) → ZnCl2(s) + H2(g)
- Why is it not advisable to use calcium in this method of preparing hydrogen? (2mrks)
- Reaction of calcium with acids is explosive
- Give the use of anhydrous calcium chloride in the U-tube (1mrk)
- To dry hydrogen gas
- Name another substance that could serve the same purpose as anhydrous calcium chloride (1mrk)
- Conc.Sulphuric VI acid or Calcium Oxide.
- Name the method used to collect gas X (1mrk)
- Upward delivery/downward displacement of air
- Identify gas X (1mrk)
- The grid below shows part of the periodic table. Use it to answer the questions that follow.
- Which of the elements has the largest atomic radius? Explain (2mrks)
- Q – Has the highest number of occupied energy levels
- Identify the most reactive metal. Explain (2mrks)
- Q – Has the largest atomic radius thus valency electrons loosely held
- Name the chemical family to which P and Q belong. (1mrk)
- Alkali metals
- Compare the atomic radius of S and U. Explain (2mrks)
- Has large atomic radius than U because U has a higher nuclear charge than S
- Select an element that does not form an ion. Explain (2mrks)
- V/W
- It is stable
- Give the formula of one stable cation with an electron arrangement of 2.8.8 (1mrk)
- Q+1
- Which of the elements has the largest atomic radius? Explain (2mrks)
- Define the term isotope (1mrk)
- Are atoms of the same element with the same atomic number/Number of protons but different mass number.
- Chlorine gas has a mass of 35.5. It is made up of two isotopes 3517CL and 3717CL. Determine the relative abundance of each isotope in the chlorine gas. (3mrks)
35.5 = (X ×35) + (100 – X) 37
100
100 × 35.5 = 35x + 3700 – 37x
100
35.5 = -2x + 3700
3550 = -2x + 3700
2x = 3700 - 3550
2x = 150
2 2
x = 75
75% & 25%
- Define the term isotope (1mrk)
- Write a balanced equation for the decomposition of the following solids (3mrks)
- Though Sodium and aluminium are in the same period and are both metals, aluminium is a better conductor of electricity. Explain (2mrks)
- Conductivity increases with increase in the number of delocalized electrons. Aluminium has more electrons than sodium.
- List any five uses of oxygen gas` (5mrks)
- Used in hospitals by patients with breathing difficulties.
- Used by mountain climbers and deep sea divers.
- Used to burn fuels.
- Used as a reactant in fuel cells.
- During steel making, Oxygen is used to remove iron impurities.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 2 Term 3 Opener Exams 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students