QUESTIONS
-
- A form one had a mixture of ethanol and water. Ethanol has a boiling point of 78°C while water has a boiling point of 100°C. The student then set up the apparatus below to separate the mixture.
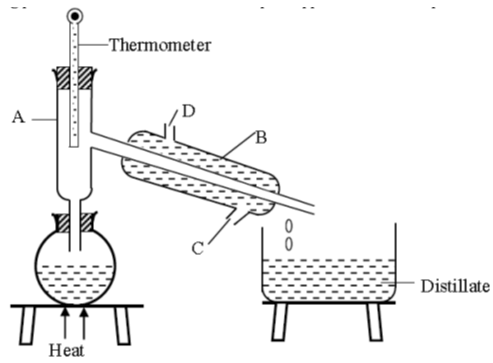
- Name the piece of apparatus labelled B. (1 mark)
- What is the purpose of the thermometer in the set up? (1 mark)
- Name the part labelled A and state its function (2 marks)
- Which liquid was collected first? Explain (1 mark)
- What is the name given to the above method of separating mixtures? (1 mark)
- What property of the components of the mixture makes it possible for the components to be separated by the method? (1mark)
- State one applications of the above method of separation. (1 marks)
- A form one had a mixture of ethanol and water. Ethanol has a boiling point of 78°C while water has a boiling point of 100°C. The student then set up the apparatus below to separate the mixture.
-
- Name the most suitable method you can use to separate;
- Xanthophyll and chlorophyll in green leaves. (1 mark)
- Oil from simsim seeds. (1 mark)
- A student set-up an experiment as shown below. Moist iron wool was placed in a boiling tube and inverted over water.
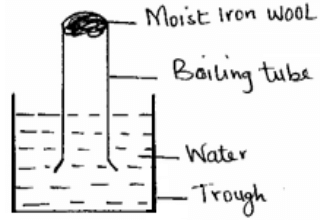
- What was observed after two days? (1 mark)
- Explain the observations. (1 mark)
- Give two factors that accelerate rusting (2marks)
-
- Give two differences between luminous and non-luminous flames. (2 marks)
- How is the non-luminous flame produced? (1 mark)
-
- Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice cream venders. (1 mark)
- Name one piece apparatus used to measure volume of gases. (1 mark)
- Draw a diagram of a deflagrating spoon. (1 mark)
- Name the most suitable method you can use to separate;
- The diagram below shows the process of manufacturing sodium carbonate using Solvay process. Study it and answer the questions that follow.
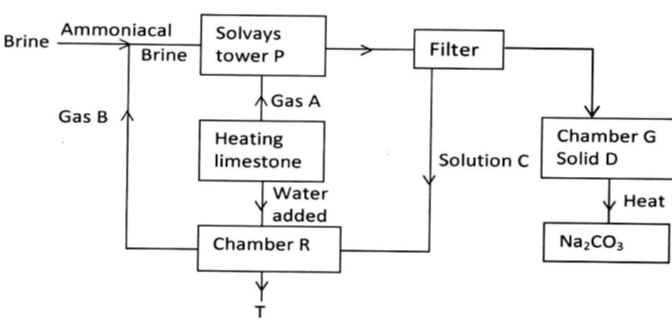
- Name gases A and B. (2mks)
- Name liquid C and solid D. (2mks)
- Write equations of the reactions in: (2mks)
Tower P:
Chamber R: - Give two uses of sodium carbonate (2marks)
- Give two substances that are recycled in Solvay process. (2marks)
- Suggest a suitable location of Solvay plant and give a reason for your answer (2marks)
-
- When extinguishing a fire caused by burning kerosene, carbon (IV) oxide is preferred to water. Explain. (2 marks)
- Write the formula of the oxide of carbon which is „silent killer‟. (1 mark)
- The grid below shows part of the periodic table. Use it to answer the questions that follow. (The letters do not represent the actual symbols.)
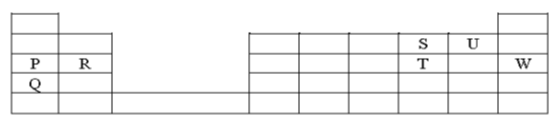
- Which of the above elements has the largest atomic radius? Explain?(2 mark)
- Identify the most reactive non-metal. Explain (2 marks)
- Write the electron configuration of ions of;
- Element S (1mark)
- Element Q (1 mark)
- Compare the atomic radius of P and R (1 mark)
- Given that the atomic mass of W is 40 write down the composition of its nucleus (2 mark)
- Write the formula of the compound formed when P and S react (1 mark)
- Give the family name to which element R belong (1 mark)
- Element X forms an ion with the formula X3- with electron configuration of 2.8. On the grid above, show the position of element X (1 mark)
- Compare the electrical conductivity of the compound formed between P and U and element Q (2 marks)
- Give one use of element P (1mark)
- Name the particles that are responsible for electrical conductivity in :( 2marks)
- Solids
- Melts
- Give two properties of graphite that make it suitable for use as an electrode (2marks)
- What is a binary electrolyte? (2marks)
- The figure below shows a set-up used in electrolysis of lead (II) iodide.
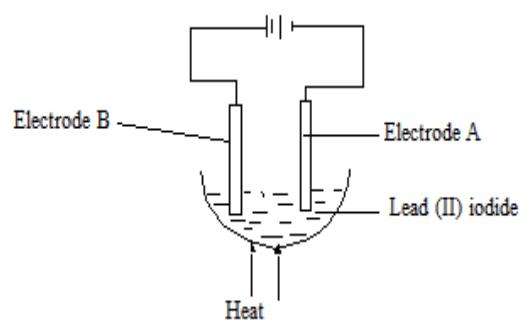
- Why is heating necessary? (1mk)
- Write the equation for the reaction that occurs at the cathode. (1mk)
- At which electrode does reduction occur? (1mk)
- Give two areas in which electrolysis process is applied? (2marks)
-
- Distinguish between coordinate bond and covalent bond? (2marks)
- Using dots (•) and crosses (x) to represent electrons in the outermost energy level, show the bonding in the following compounds.
- PCl3 (3 mark)
- Illustrate bonding in carbon (II) oxide using dot (•) and cross (x)
(C – 6, O – 8). (3 marks)
- An element X is atomic number 3, relative atomic mass 6.94 and consists of two isotopes of mass numbers 6 and7respectively.
- What is the mass number of the more abundant isotope of X? (1mk)
- Calculate the relative abundance of each of the isotopes. (2mks)
- Distinguish between ionization energy and electron affinity. (2 marks)
- The atomic number of Q and R are 9 and 17 respectively. Compare the electron affinity of Q and R . Explain. (1 mark)
-
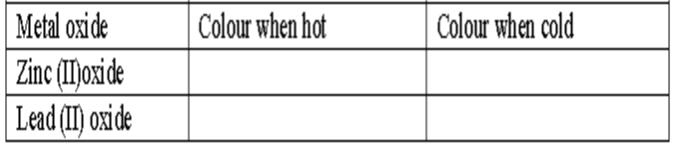
- What is the colour of the following.(2marks)
- A mixture contains sodium chloride, ammonium chloride and copper (II) oxide. Describe how each substance can be obtained from the mixture. (3mks)
- What is the colour of the following.(2marks)
MARKING SCHEME
-
- A form one had a mixture of ethanol and water. Ethanol has a boiling point of 78°C while water has a boiling point of 100°C. The student then set up the apparatus below to separate the mixture.

- Name the piece of apparatus labelled B. (1 mark)
- Liebig condenser
- What is the purpose of the thermometer in the set up? (1 mark)
- It helps in determining the boiling range so that pure ethanol is obtained. When liquids boil, the temperatures remain constant.
- Name the part labelled A and state its function (2 marks)
- Part A: Fractionating column.
- It increases the surface area for condensation of the substance whose boiling point has not been reached. ½
- Provides a surface in which vapour condenses into liquid and the liquid redistills.½
- Which liquid was collected first? Explain (1 mark)
- Ethanol was collected first ½
- It has a lower boiling point 780C, hence it boils first. ½
- What is the name given to the above method of separating mixtures? (1 mark)
- Fractional distillation 1
- What property of the components of the mixture makes it possible for the components to be separated by the method? (1mark)
- Have different but close boiling points.
- State one applications of the above method of separation. (1 marks)
- Manufacture of spirits
- Fractional distillation of crude oil.
- Obtaining oxygen from liquid air. (Any 0ne which are correct 1 marks)
- Name the piece of apparatus labelled B. (1 mark)
- A form one had a mixture of ethanol and water. Ethanol has a boiling point of 78°C while water has a boiling point of 100°C. The student then set up the apparatus below to separate the mixture.
-
- Name the most suitable method you can use to separate;
- Xanthophyll and chlorophyll in green leaves. (1 mark)
- Chromatography. (1mk)
- Oil from simsim seeds. (1 mark)
- Solvent extraction. (1mk)
- Xanthophyll and chlorophyll in green leaves. (1 mark)
- A student set-up an experiment as shown below. Moist iron wool was placed in a boiling tube and inverted over water.

- what was observed after two days? (1 mark)
- Moist iron wool had turned to brown.
- Explain the observations. (1 mark)
- The brown substance (½mk) was rust which occurred due to presence of moisture and oxygen.
- Give two factors that accelerate rusting(2marks)
- Temperature, salt and acid(any two factors)
- what was observed after two days? (1 mark)
-
- Give two differences between luminous and non-luminous flames. (2 marks)
luminous non-luminous bright yellow flame blue flame sooty non-sooty 4-regions 3-region large and wavy short and steady - How is the non-luminous flame produced? (1 mark)
- When the air hole is fully open.
- Give two differences between luminous and non-luminous flames. (2 marks)
-
- Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice cream venders. (1 mark)
- Sublimes leaving no wetness
- Name one piece apparatus used to measure volume of gases. (1 mark)
- Syringe
- Draw a diagram of a deflagrating spoon. (1 mark)
- Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice cream venders. (1 mark)
- Name the most suitable method you can use to separate;
- The diagram below shows the process of manufacturing sodium carbonate using solvay process. Study it and answer the questions that follow.

- Name gases A and B. (2mks)
- A – Carbon (IV) oxide√1
- B – Ammonia √1
- Name liquid C and solid D. (2mks)
- C – Ammonium chloride solution√1
- D – Sodium hydrogen carbonate√1
- Write equations of the reactions in: (2mks)
- Tower P:
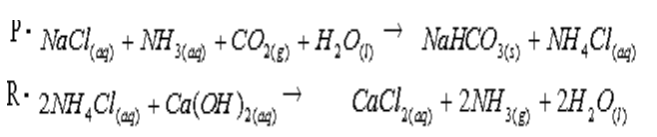
Chamber R:
- Tower P:
- Give two uses of sodium carbonate (2marks)
- Paper industry
- Manufacture of glass
- Softening of water
- Give two substances that are recycled in Solvay process.(2marks)
- Carbon (iv) oxide, water and ammonia (any two)
- Suggest a suitable location of Solvay plant and give a reason for your answer (2marks)
- Close to a river or large source of water to cool down the carbonator.
-
- When extinguishing a fire caused by burning kerosene, carbon (IV) oxide is preferred to water. Explain. (2 marks)
- Kerosene floats on water therefore it continues to burn. Carbon (IV) oxide cuts off the supply of oxygen.
- Write the formula of the oxide of carbon which is „silent killer‟. (1 mark)
- CO
- When extinguishing a fire caused by burning kerosene, carbon (IV) oxide is preferred to water. Explain. (2 marks)
- Name gases A and B. (2mks)
- The grid below shows part of the periodic table. Use it to answer the questions that follow. (The letters do not represent the actual symbols.)

- Which of the above elements has the largest atomic radius? Explain? (2 mark)
- Element Q.it has the highest number of occupied energy levels i.e four energy levels
- Identify the most reactive non-metal. Explain (2 marks)
- U.it has seven valence electrons therefore gains only one electron to attain stability
- Write the electron configuration of ions of;
- Element S (1mark)
- 2.8
- Element Q (1 mark)
- 2.8.8
- Element S (1mark)
- Compare the atomic radius of P and R (1 mark)
- P has a bigger atomic radius as compared to R. R has more protons therefore stronger nuclear force of attraction resulting in smaller atomic radius
- Given that the atomic mass of W is 40 write down the composition of its nucleus (2 mark)
- 18 protons and 22 neutrons
- Write the formula of the compound formed when P and S react (1 mark)
- P2S
- Give the family name to which element R belong (1 mark)
- Alkaline earth metals
- Element X forms an ion with the fomula X3- with electron configuration of 2.8. On the grid above, show the position of element X (1 mark)
- Compare the electrical conductivity of the compound formed between P and U and element Q (2 marks)
- The compound formed between Pand U conducts using mobile ions while G conducts through delocalised electrons
- Give one use of element P (1mark)
- Manufacture of sodium cyanide which is used in extraction of gold
- Sodium vapour is used to produce the yellow glow in streetlights (any correct use of sodium)
- Which of the above elements has the largest atomic radius? Explain? (2 mark)
- Name the particles that are responsible for electrical conductivity in :( 2marks)
- Solids
- delocalised electrons
- Melts
- Free/mobile ions
- Give two properties of graphite that make it suitable for use as an electrode (2marks)
- Cheap
- It does not react with many electrolytes
- What is a binary electrolyte? (2marks)
- One which contains only one type of cation and one type of anion
- The figure below shows a set-up used in electrolysis of lead (II) iodide.

- Why is heating necessary? (1mk)
- To melt the lead (II) iodide and form free ions (1mk)
- Write the equation for the reaction that occurs at the cathode. (1mk)
Pb2+(l) + 2e → Pb(s) - At which electrode does reduction occur? (1mk)
- Cathode (electrode A) (1mk)
- Give two areas in which electrolysis process is applied?(2marks)
- Purification of metals
- Extraction of metals such as sodium, magnesium and aluminium by electrolysis of their molten compounds
- Electroplating of metals such as iron to prevent rusting
- Why is heating necessary? (1mk)
- Solids
-
- Distinguish between coordinate bond and covalent bond? (2marks)
- In coordinate bond, the shared pair of electrons forming the bond is contributed by only one of the atom forming the bond whereas in covalent bond, the shared pair of electrons is contributed equally by the atoms forming the bond.
- Using dots (•) and crosses (x) to represent electrons in the outermost energy level, show the bonding in the following compounds.
- PCl3 (2 mark)
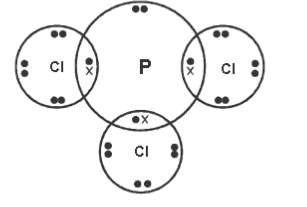
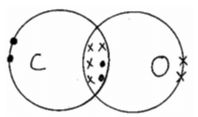
- Illustrate bonding in carbon (II) oxide using dot (•) and cross (x) (C – 6, O – 8). (2 marks)
- PCl3 (2 mark)
- Distinguish between coordinate bond and covalent bond? (2marks)
-
- An element X is atomic number 3, relative atomic mass 6.94 and consists of two isotopes of mass numbers 6 and7respectively.
What is the mass number of the more abundant isotope of X? (1mk)- 7
- Calculate the relative abundance of each of the isotopes. (2mks)
- Let % of isotope 7 be x
therefore: % of isotope 6 = 100 - x
7x+ 600 - 6x= 694
x = 694 – 600
x = 94%
Isotope – 7 is 94%
Isotope – 6 is 6%
- Let % of isotope 7 be x
- Distinguish between ionization energy and electron affinity. (2 marks)
- Ionisation energy – is the energy required to remove one mole of electrons from one mole of atoms in gaseous state
- While electron affinity is the energy required by an atom to acquire an electron in the outermost energy level in gaseous state
- The atomic number of Q and R are 9 and 17 respectively. Compare the electron affinity of Q and R . Explain. (1 mark)
- R is higher / greater ½ that Q because Q is a smaller atom therefore its nucleus ½ attracts electrons strongly.
- An element X is atomic number 3, relative atomic mass 6.94 and consists of two isotopes of mass numbers 6 and7respectively.
-
- What is the colour of the following.(2marks)
metal oxide color when hot color when cold zinc(II)oxide yellow white lead(II)oxide red yellow - A mixture contains sodium chloride, ammonium chloride and copper (II) oxide. Describe how each substance can be obtained from the mixture. (3mks)
- First heat the mixture Ammonium chloride sublimes
- Add water to the remaining mixture)
- Sodium chloride will dissolve Copper (II) oxide does not dissolve
- Filter off the copper (II) oxide
- Heat the filtrate to evaporate sodium chloride
- What is the colour of the following.(2marks)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 2 End of Term 3 Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

