QUESTIONS
Attempt All Questions.
-
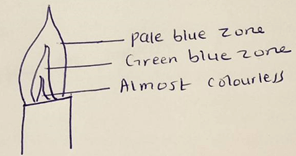
- When the air-hole is fully opened, the Bunsen burner produces a non-luminous flame.
Explain(2 mark) - Draw a labelled diagram of a non-luminous flame. (3 marks)
- When the air-hole is fully opened, the Bunsen burner produces a non-luminous flame.
- The diagram below shows some parts of a Bunsen burner
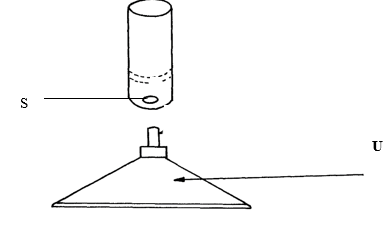
Name parts S and U and state their functions. (4 marks) - A mixture of hexane and water was shaken and left to separate as shown in the diagram below:
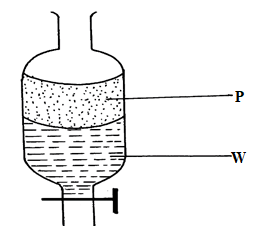
Name the above method of separation. (1 mark) - The set-up below is used to investigate the properties of hydrogen.
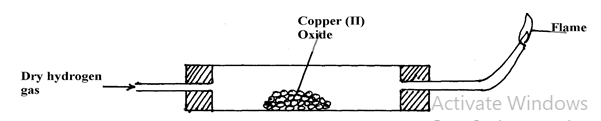
- On the diagram, indicate what should be done for the reaction to occur (1 mark)
- Hydrogen gas is allowed to pass through the tube for some time before it is lit. Explain (2mark)
- Write a chemical equation for the reaction that occurs in the combustion tube. (1 mark)
- When the reaction is complete, hydrogen gas is passed through the apparatus until they cool down. Explain (2mark)
- What property of hydrogen is being investigated? (1 mark)
- What observation confirms the property stated in (v) above? (2mark)
- Why is zinc oxide not used to investigate this property of hydrogen gas? (1mark)
- Explain why an atom is said to be electrically neutral. (2 marks)
- The table below gives the atomic numbers of elements W, X, Y, and Z. The letters do not represent the actual symbols of the elements.
Element W X Y Z Atomic Number 4 10 11 12 - Which one of the elements is least reactive? Explain. (1mark)
-
- Which two elements belong to the same group? (1mark)
- Give the formula of the Z sulphate. (1mark)
- give two pairs of elements that belong to the same period (2mks)
- in an experiment to separate a mixture of ethanol and water. Ethanol boils at 78ºC and water 100ºC. A student set up the apparatus shown below.
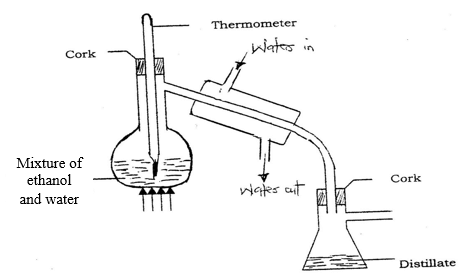
- Identify two mistakes in the set – up. (2marks)
- What method would the student use to test the purity of the distillates obtained? (1mark)
- Name the above method of separation. (1mark)
- State the two applications of the above process. (2 marks)
- Which liquid will distill off first. Explain. (2 marks)
- The grid below shows part of the periodic table. Use it to answer the question that follows. (Letters are not actual symbols of elements.)
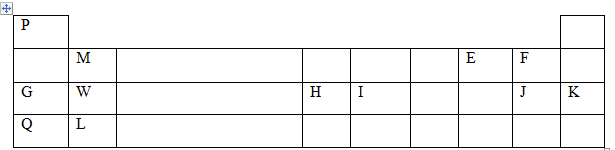
- Write the electronics configuration of the following element. (2mk)
- E
- L
- Give the formula of one stable ion with an electron arrangement of 2:8 which is; (2mark)
- Negatively charged.
- Positively charged
- Select two alkali metals from the above periodic table (2 mark)
- Compare the atomic radius of G and Q, give a reason. (2mark)
- Write the chemical equation between W and oxygen. ( 1 mark)
-
- State three observations made when a piece of element G is placed on water, (3marks)
- Write a chemical equation between element G and water. (2mark)
- Write the electronics configuration of the following element. (2mk)
-
- What is meant by the terms?
- Atom; (2mk)
- Isotopes? (2mk)
- The formula for a sulphate of titanium is Ti2 (SO4)3. Write the formula of its chloride?(1mk)
- Calculate the relative atomic mass of Neon given that it exist as;
90.92% 2010Ne , 0.26% 2110Ne , 8.82% 2210Ne, ( 3 marks)
- What is meant by the terms?
- The diagram below shows students set-up for the preparation and collection of oxygen gas
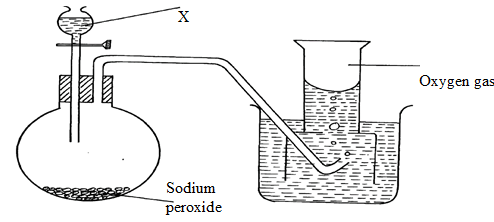
- Name substance X used ( 1 mark)
- Write an equation to show the reaction of sodium peroxide with the substance named in 1(a) (1 mark)
- State 3 uses of oxygen gas. ( 3 marks)
- Name the method of collection shown above ,explain. ( 2marks)
- The following table shows solutions with their pH values. Use it to answer the questions that follow.
Solution A B C D E pH 1 7 14 9 5 - Identify a solution which ( 2marks)
strong acid
strong base. - Which solution is used in the manufacture of anti acid tablets (1 marks)
- State a commercial indicator that cannot be used to classify the solutions into, acids, base or neutral. Explain your answer. ( 2marks)
- Identify any two solution that react to form salt and water only. (1 marks)
- Identify a solution which ( 2marks)

MARKING SCHEME
-
- There is complete combustion of gases
- S - Airhole
It allows air to the chimney
U - Base
It offers the support - Separating funnel

- To drive out air, because when hydrogen and air mixes, when lit, an explosion can occur
- CuO(s) + H2(g) → Cu(s) + H2O(g)
- To allow the copper to cool to prevent oxidation by air
- Reducing agent
- Black copper (II) oxide turns to brown copper
- Zinc is more reactive Hydrogen
- The number of protons are equal to the number of electrons
-
- X is stable
-
- W and Z
- ZSO4
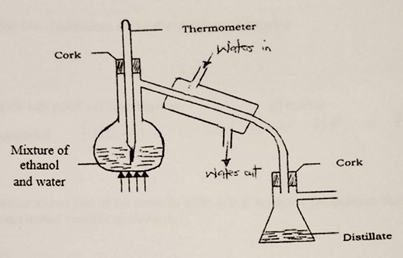
- The thermometer has been dipped inside the mixture
Water in and out is wrongly places; inverted - Determine boiling point, melting point, density
- Simple distillation
- Ethanol. It has lower b.p of 78ºC
- The thermometer has been dipped inside the mixture
-
-
- 2.6
- 2.8.8.2
-
- E2-, F-
- G+, W2+, H3+
- G and Q
- Q has a larger atomic radius than G
Q has 4 occupied energy levels while G has 3 - WO
-
-
- Floats on water
- Melts into silvery ball
- Hissing sound is produced
- G(s) + H2O(I) GOH(aq) + H2(g)
-
-
-
-
- It is the smallest particle of an element that can take part in a chemical reaction
- Atoms of the same element with the same atomic number but different mas number
- TiCl
- R.AM = 90.92 x 20 + 0.26 x 21 + 8.82 x 22
100
= 2017.9
100
= 20.179
-
-
- Water
- 2Na2O2(s) + H2O(I) 4NaOH(aq) + O2(g)
-
- Oxygen mixed with hydrogen or acetylene used in welding
- Oxygen is used in hospital with patients with breathing problems
- Over water, it is slightly soluble in water/ insoluble in water
-
- A
C - D
- Methyl orange - It shows distinct colour in acid, base and indicator
Litmus paper - A and B
A and D
D and E
C and E
(Any)
- A
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 2 Term 2 Opener Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

