QUESTIONS
- The figure below shows a frame obtained from a Bunsen burner.
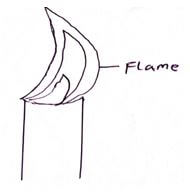
- Name the type of a flame. (1mks)
- State one shortcoming of using this flame in heating substances in the laboratory. (1mk)
- It is always advisable to leave the burner with the air hole closed when it is lit bit not in use. Explain. (2mks)
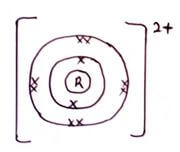
- An element 24R
12- To which chemical family does it belong? (1mk)
- Write the electron arrangement of the atom (1mk)
- Draw the structure of its Ion. (1mk)
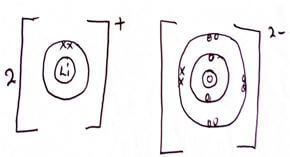
- Use dot (.) and cross (x) to show the bonding in Lithium Oxide. (2mks)
- Excess magnesium ribbon was burnt in air to form a white solid mixture. Write two equations to show the formation of the white solid mixtures. (2mks)
- State two laboratory rules that should be followed to avoid contamination and wastage of chemicals (2mks)
- State two application of chromatography. (2mks)
- A student planned some hydrogen peroxide in a test tube then added a small amount of manganese (IV) Oxide - . A glowing splint was then brought near the mouth of the tube.
- State the observation made on the glowing splint. (1mk)
- What is the role of the Manganese (IV) Oxide (1mk)
- Give two use of the gas produced. (2mks)
- Define the flowing terms as used in salts
- Deliquescence (1mk)
- Hygroscopic. (1mk)
- Hygroscopy. (1mk)
- Complete the following equation on effect of heat on Nitrates and carbonates. (4mks)
NaNO3(s) →
Zn (NO3)2 →
Ag NO3(s) →
CaCO3(s) → - Explain why graphite is a non-metal made up of element carbon and yet conducts electric current. (2mks)
- Explain why lead (ii) bromide does not conduct electric current in solid state but contact in molten state. (1mk)
- A certain element Y has atomic number 15 and mass number of 31.
Calculate this number of Neutrons in the element. (1mk)
MARKING SCHEME
- The figure below shows a frame obtained from a Bunsen burner.

- Name the type of a flame. (1mks)
non-luminous flame - State one shortcoming of using this flame in heating substances in the laboratory. (1mk)
It uses a lot of laboratory gas in burning. - cannot be used for lighting purposes since it produces very little light. - It is always advisable to leave the burner with the air hole closed when it is lit bit not in use. Explain. (2mks)
The flame is bright yellow and big, hence can easily be seen to avoid accident.
- Name the type of a flame. (1mks)
- An element 2412R
- To which chemical family does it belong? (1mk)
Alkaline earth metals. - Write the electron arrangement of the atom (1mk)
2.8.2 - Draw the structure of its Ion. (1mk)
- To which chemical family does it belong? (1mk)
- Use dot (.) and cross (x) to show the bonding in Lithium Oxide. (2mks)
- Excess magnesium ribbon was burnt in air to form a white solid mixture. Write two equations to show the formation of the white solid mixtures. (2mks)
Magnesium gives up two electrons to oxygen atoms to form this powdery product. This is an exothermic reaction. An exothermic reaction is a term that describes a chemical reaction in which there is a net release of energy (heat).
2Mg (s) + O2 (g) → 2MgO(s)
3Mg(s) + N2(g) → Mg3N2 (s)
- State two laboratory rules that should be followed to avoid contamination and wastage of chemicals (2mks)
Do not return chemicals to their original packaging. ...
Keep chemical containers closed. ...
Never use a wrong or an unmarked reagent. ...
Never put spatulas, stirrers or other objects into a storage container for chemicals.
Label all containers carrying chemicals.
Turn off water and gas taps when not in use.
Use
Always work on a clean bench. - State two application of chromatography. (2mks)
1) It is used to separate solution of coloured substances. 2) It is used in forensic sciences to detect and identify trace amount of substances in the contents of bladder and stomach. 3) It is used to separate small amount of products of chemical reaction.
- In sports to identify banned substances
- To test purity of drugs in Pharmacy
- Identify contaminants in food and drunks
- Identify harmful substances in cosmetics. - A student planned some hydrogen peroxide in a test tube then added a small amount of manganese (IV) Oxide - . A glowing splint was then brought near the mouth of the tube.
- State the observation made on the glowing splint. (1mk)
It relight a glowing splint. - What is the role of the Manganese (IV) Oxide (1mk)
Serves as a catalyst. - Give two use of the gas produced. (2mks)
- Breathing aid by patients with respiratory problems
- Ox hydrogen flame is used in welding and cutting metals
- Mixed with hilum for deep sea diving and mountain climbing.
- State the observation made on the glowing splint. (1mk)
- Define the flowing terms as used in salts
- Deliquescence (1mk)
It is the process in which salts when being exposed in atmosphere absorb water and forms a solutions. - Hygroscopic. (1mk)
These rare salts when exposed in the atmosphere they absorb water but does not dissolve to form solutions. - Hygroscopy. (1mk)
This is the process in which when some of the slats exposed in the atmosphere absorb water but does not form solutions.
- Deliquescence (1mk)
- Complete the following equation on effect of heat on Nitrates and carbonates. (4mks)
NaNO3(s) → NaNO2 (s) + O2
Zn (NO3)2 → ZnO + 4NO2 + O2
Ag NO3(s) → Ag + NO2 +O2
CaCO3(s) → CaO (s) + CO2 (g) - Explain why graphite is a non-metal made up of element carbon and yet conducts electric current. (2mks)
In graphite the carbon uses 3 – outermost electrons to bond to form graphite while one remain for delocalization. - Explain why lead (ii) bromide does not conduct electric current in solid state but contact in molten state. (1mk)
In molten state the lead (ii) bromide has mobile Ions that contact electric current. - A certain element Y has atomic number 15 and mass number of 31.
Calculate this number of Neutrons in the element. (1mk)
31-15= 16 neutrons
Download Chemistry Questions and Answers - Form 2 Mid Term 2 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

