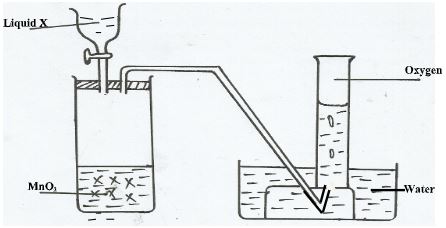
- The diagram below shows oxygen can be prepared in a laboratory. Study it and answer the questions that follow.
- Name the liquid X (Imark)
- Write a balance chemical equation to shows how oxygen is produced in the boiling tube. (1mark)
- Give one industrial use of oxygen(1mark)
- Explain the observation made in (b) above (Imark)
- Metal Xreacts with cold water slowly while Y does react with neither cold water nor hot water. Metal Z react with both cold water and hot water vigorously and explosively respectively. Arrange these metals in order of increasing reactivity (2mark)
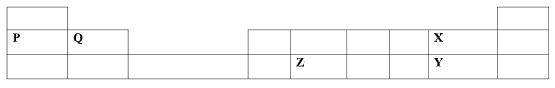
- The grid below shows part of the periodic table. The letters do not represent the actual symbols of elements.
- Compare
- Atomicradii ofP and Q(1mark)
- Reactivity of X and Y (Imark)
- Write the chemical formula of a compound formed by Q after reacting with X. (1mark)
- Compare
- Using dots(.) and crosses (x) show the bonding
- NH4+ (1mark)
- H2O (1mark)
-
- Name two allotropes of carbon. (1mark)
- Explain why diamond is very hard. While graphite is very soft. (2marks)
- Carbon (II) oxide is a'silent killer'. Explain. (2marks)
- Name the following processes;
- When anhydrous calcium chloride is left in an open beaker overnight a solution was formed. (1mk)
- When sodium carbonate decahydrate crystals are left in an open beaker for some days it turned into a powder. (1mk)
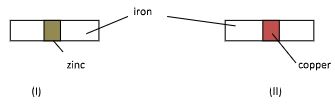
- In an experiment, two pieces of iron sheets were wrapped in each case with zinc and copper metal sheets as shown below. They were left in the open for some months.
- State and explain the observations made in the experiments;
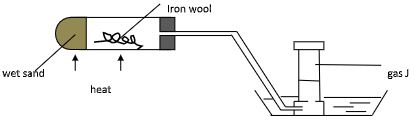
- Study the diagram below and answer the questions that follow.
- Name gas J (1mk)
- Explain why its important to heat the wet sand before heating the Iron wool. (1mk)
- Name the product formed in the combustion tube. (1mk)
- Solutions can be classified as acids, bases or neutral. The table below shows solutions and their pH values
Solution Ph-values K 1.5 L 7.0 M 14.0 - Select any pair that would react to form a solution of pH 7 (1 Mark)
- Identify two solutions that would react with aluminium hydroxide. Explain (2 Marks)
- Oxygen gas can be prepared in the laboratory by heating potassium nitrate.
- Write the equation of reaction to show the decomposition of potassium nitrate (1 Mark)
- State two physical properties of oxygen gas (1 Mark)
- Outline one industrial use of oxygen gas (1 Mark)
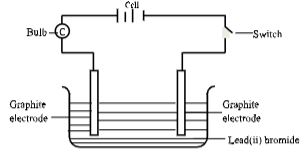
- Study the set-up below for electrolysis of molten lead(ii) bromide using grahite electrodes.
- Write ionic equations for reactions that took place at
- Anode (1⁄2 Mark)
- Cathode (1⁄2 Mark)
- State the observation made at each electrode
- Anode (1⁄2 Mark)
- Cathode (%1⁄2 Mark)
- State and explain the observations made on the electrolyte (1 Mark)
- Write ionic equations for reactions that took place at
-
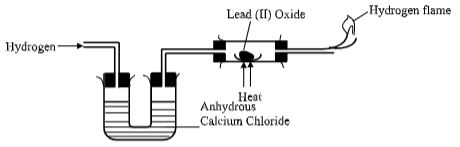
- State the role of the following parts during fractional distillation of a mixture of water and ethanol
- Glass beads in the fractionating column (1 Mark)
- Fractionating column (1 Mark)
- State any one application of fractional distillation (1 Mark)
- State the role of the following parts during fractional distillation of a mixture of water and ethanol
- Write an equation for the reaction that takes place in the tube (1 Mark)
- What property of hydrogen makes this reaction possible? (1 Mark)
- What would you expect to happen, if sodium oxide (Na,O) was used instead of Lead (II) oxide? (1 Mark)
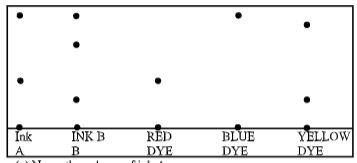
- The chromatogram of two inks and three dyes is drawn below.
- Name the colours of ink A (1 Mark)
- Suggest how separated components can be recovered (Mark)
- Suggest two reasons why separations occur in this method (1 Mark)
-
- Why is reaction between calcium and dilute sulphuric (VI) acid not used in preparation of hydrogen gas (2 Marks)
- Calcium is an element in period 2, what do members of the period have in common? (1 Mark)
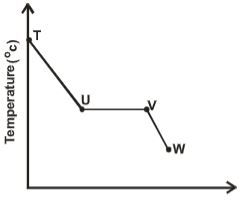
- The sketch below shows a graph of temperature against time obtained when a gaseous substance was cooled.
Explain what happens to the gaseous substance between:- T and U (1 mack)
- U and V (1mark)
- V and W (1mark)
- Diamond and graphite are both allotropes of carbon. Explain why graphite is a good conductor of electricity while diamond is not. (2 marks)
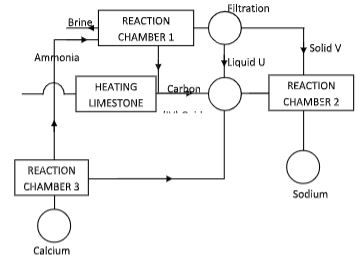
- The figure below shows the stages in the manufacture of sodium carbonate. Study the diagram below and use it to answer the questions that follow.
- Name three starting materials in the manufacturer of sodium carbonate.( 1%, marks)
- Which substances are recycled in this process? (1, marks)
- Identify the chambers in which the recycled substances are regenerated.( 1, marks)
- Name the substances U and V. (1 marks)
- Give an equation for the reaction which occurs:
- In the reaction charnber 1 (1 rnark)
- When solid V is heated. (1 mark)
- In the reaction chamber 3.( 1 mark)
- State one commercial use for
- Sodium carbonate. (1 mark)
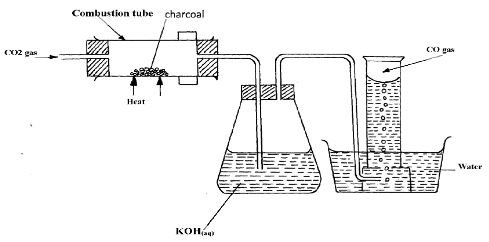
- The set-up below was used to prepare dry carbon (II) Oxide gas. use it to answer the questions. below it:
-
- State two mistakes committed in the set-up arrangement above(2 marks)
- The student produced carbon (IV) oxide gas from the reaction between Lead (II) Carbonate and dilute hydrochloric acid. The gas was produced for a short time and the reaction came to a stop. Explain (2 marks)
- Write the equation for the reactions taking place in the combustion tube and the conical flask: (2 marks)
- Combustion tube:...
- Conical flask
- State one use of carbon (IV) Oxide gas apart from fire extinguisher(1 mark)
- Give two properties that make carbon (IV) Oxide to be used as fire extinguisher (2 mark)
- PbO+CO → Pb+CO
- Which property of carbon (II) Oxide is demonstrated by the above equation? (1 mark)
- Aluminium carbonate does not exist. Give a reason (1 mark)
- Ammonium carbonate decomposes when heated. Write a chemical equation to represent this decomposition (1 mark)
-
MARKING SCHEME
-
- Hydrogen peroxide
- 2H2O2(aq) MnO→2H20(l) +02(g)
Balance with correct state units
Balance with wrong/missing units - welding
Purification of iron
- Z, X, Y must be correct in order
-
-
- Q is smaller than P/P is bigger than Q
- X is more reactive than Y
- QX
-
-
- Graphite and diamond
- Diamond has giant atomic structure with tetrahedral structure while graphite has hexagonal planes joint by weak van der waals forces.
-
- When anhydrous calcium chloride is left in an open beaker overnight a solution was formed. (1mk)
- deliquescence
- When sodium carbonate decahydrate crystals are left in an open beaker for some days it turned into a powder. (1mk)
- efflorescence ✓
- When anhydrous calcium chloride is left in an open beaker overnight a solution was formed. (1mk)
-
- No rusting. Zinc is above iron in the reactivity series
- Rusting occurs. Iron is more reactive than copper
-
-
- Name gas J
- hydrogen(1mk)
- Explain why its important to heat the wet sand before heating the iron wool. (1mk)
- To drive out air in the tubb
- Name the product formed in the combustion tube. (1mk)
- Tri-iron tetraoxide
-
-
- K and M✓1
- K1⁄2 and M1⁄2. This is because K is acidic and M is basic and aluminium hydroxide being amphoteric would react with both. ✓1
-
- 2KNO2(s) –→ 2KNO +0:1
-
- Colourless/odourless gas
- Less dense than air
- Used in welding when mixed with acetylene gas ✓ 1
-
-
- Br—→ Br: +2e-1⁄2 (Anode)
- Pb2+2e-→Pb Cu1⁄2 (Cathode)
- Anode - Copper anode dissolves or decreases in mass/1⁄2
Cathode - Copper electrode increases in size/mass✓1⁄2 or copper metal is deposited there - Blue colour of CuSO, remains the same 1⁄2, because the Cu discharged at cathode were replaced when Cu dissolves.
-
-
-
- Increases the surface area for condensation 1⁄2 process.
- It allows water vapour to condense1⁄2 into liquid and flow back into the flask before the boiling point of water is reached
- During oil refinery, crude oil is separated into a number of fractions ✓1
-
-
- PbO+H ——→ Pb(s)+H2O(l)✓ 1
- Reducing property
- No reaction1⁄2, sodium metal is more reactive than hydrogen, hence hydrogen cannot reduce oxide to element sodium
-
- Red and blue ✓ 1⁄2
- By solvent extraction
-
- Unequal solubilities
- Different absorption abilities
-
- Due to formation of insoluble layer of CaSO. which coats the metal hence preventing further reaction from taking place.
- All have same no. of energy level
-
- TU-The gaseous substance cools by losing heat
- UV-the substance changes to liquid state at constant temperature
- VW-The substance (liquid) cools by losing heat
- In graphite, one electron per carbon atom is mobile and delocalized over the whole layer, while in diamond all the four electrons of an atom are used is covalent bonding, hence no delocalized electrons that can conduct electricity
-
-
-
- Ammonia gas =1
- Calcium carbonate. [1
- Brine 1 or Concentrated sodium chloride.
- Coke
(Any three materials)
-
- Carbon (IV) oxide. 1
- Ammonia gas. 1
- Water
-
- Chamber 3
- Chamber 2
-
- U-Ammonia chloride 1
- V-Sodium hydrogen carbonate. 1
-
-
-
- HN (g) + HO(l) + CO(g) + NaCl(aq) ———→NH,Cl(aq) + NaHCO3(s)
OR - NH3(g) + H,2{l) + CO(g) ———→NH4HOO3 (aq)
- NH4HCO3(aq) + NaCl(aq) ————→ NH4Cl(aq) + NaHCO3(s)
- HN (g) + HO(l) + CO(g) + NaCl(aq) ———→NH,Cl(aq) + NaHCO3(s)
- NaHCO, ————→Na2C3(s)+ CO2(g) + H2O(l)
- Ca(OH)2(s) + 2NH2Cl(aq) ————→CaCl2NH3(g) + 2H20(1)
-
-
- Manufacture of glass.
- Softening of hard water.
- Manufacture of papers.
- Manufacture of soap.
- Refining of metals.
-
-
-
-
- The gas is collected over water
- The gas is not passed through a drying agent
- PbCl, is formed which is insoluble hence prevents contact between the carbonate and the acid
- CO2(g) + C(s) ———→ 2C0(g)
CO2+2NaOH ————→Na2CO3(aq)+HO(l) -
- Solid CO, used as a refrigerant
- Used in making aerated drinks
- Solid CO, is used in cloud-seeding
- CO, used as an ingredient/air material in solvary process
- Denser than air
-Does not support combustion (burning)
-
- Reducing Property
- Al2(CO3)3 hydrolyses in water/moisture forming Hions which reacts with the carbonate and dissolves
- (NH4)2 CO3(s) ————→ NH3(g) +CO2(g) + H2O(g)
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 2 Term 2 Opener Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students