INSTRUCTIONS TO CANDIDATES
- Answer ALL questions
- You are NOT allowed to start working with the apparatus for the first 15 minutes of the time allowed for this paper.This time is to enable you read through the question paper and make sure you have all the chemicals and the apparatus that you may need.
- ALL working must be clearly shown where necessary.
- Mathematical tables and electronic calculators may be used

QUESTIONS
Question 1 (20 MARKS)
You are provided with:
- Solution H which is potassium manganate (VII) solution.
- Solution X which is dilute solution of hydrogen peroxide.
- Solution N which is 0.02M ammonium iron (II) sulphate solution.
You are required to:- Standardize solution H using solution N.
- Use the standardized solution H to determine the concentration of solution X.
Procedure 1
- Fill the burette with solution H.
- Pipette 25cm3 of solution N and transfer it into a conical flask.
- Titrate solution N against solution H until a permanent pink colour just appears.
- Record the results in table 1 below.
- Repeat the titration two more times to complete the table.
- Table 1 (3mks)
I II III Final burette reading (cm3) Initial burette reading (cm3) Volume of solution H used (cm3) - Determine the average volume of solution H used. (1mk)
- Calculate;
- The number of moles of solution N in 25cm3. (2mks)
- The number of moles of solution H that reacted given the equation for the reaction is (2mks)
MnO4-(aq) + 5Fe2+(aq)+ 8H+(aq) → Mn2+(aq) + 5 Fe3+(aq) + 4H2O(l) - The concentration of H in moles per litre. (2mks)
- Table 1 (3mks)
Procedure II
- Fill the burette with solution H.
- Using a clean pipette, place 25cm3 of solution X into a conical flask.
- Add 10cm3 of 1Msulphuric acid and shake well.
- Titrate using solution H until a permanent pink colour just appears.
- Record the reading in table II below.
- Repeat the titration two more times to complete the table.
- Table II (3mks)
I II III Final burette reading (cm3) Initial burette reading (cm3) Volume of solution H used (cm3) - Determine the average volume of solution H used. (1mk)
- Calculate;
- The number of moles of solution H used. (2mks)
- The number of moles of solution X in 25cm3 if the equation for the reaction is;(2mks)
2MnO42-(aq) + 6H+(aq) + 5H2O2(l) → 2Mn2+(aq) + 8H2O(l) + 5O2(g) - The concentration of solution X in moles per litre. (2mks)
- Table II (3mks)
QUESTION 2 (11 MARKS)
You are provided with the following;
- Solid Y
- Sodium chloride solution
- Potassium chloride solution
- Calcium chloride solution
- You are required to identify the cations present in solid Y.
The following notes will assist in making the correct observations and inferences.
Cations are positively charged ions, majority of which are metal ions. Cations can be tested using one or a combination of the following methods;
- Flame tests
- Some cations burn with flames that have distinct colours.
- Carrying out precipitation reactions using the following;
- Sodium hydroxide
- Aqueous ammonia
- Anions such as CO32-, SO42-, Cl- and SO32-
- Precipitates are formed as a result of formation of insoluble salts or metal hydroxides.
- The colour of the precipitate should be noted down when writing the observations.
- Incase a white precipitate is expected and not observed, then one should record that there is no white precipitate but NOT no observation.
- It is important to note that hydroxide of zinc, lead and aluminium are amphoteric thus can react with sodium hydroxide which is alkaline. Another thing to note is that zinc hydroxide and copper (II) hydroxide dissolve in excess aqueous ammonia due to formation of complex ions.
a) Procedure
Carry out the tests below and record your observations and inferences in the spaces provided.
- Place all of the solid Y provided in a boiling tube. Add about 10cm3 of distilled water and shake well. Use about 2cm3 of the resulting solution to carry out tests (i)to (iii) below. Reserve the remaining portion for test (b).
Observations Inference (1mk) (1mk) - To the first portion, add aqueous sodium hydroxide dropwise until in excess.
Observations Inference (1mk) (1mk) - To the second portion, add aqueous ammonia dropwise until in excess.
Observations Inference (1mk) (1mk) - To the third portion, add about 1cm3 sodium chloride solution.
Observations Inference (1mk) (1mk)
- To the first portion, add aqueous sodium hydroxide dropwise until in excess.
- Procedure
Clean a glass rod and rinse it with distilled water. Dry the glass rod on a Bunsen burner flame. Allow it to cool. Dip it in a little sodium chloride solution and burn it strongly with a non-luminous Bunsen burner flame. Note the colour of the flame and record it in table III below. Clean the spatula thoroughly and repeat the procedure using each of the other solutions and complete table III.- Table III (2mks)
Solution Color of Flame Sodium chloride Potassium chloride Calcium chloride Solution Y - From table III, suggest the cation that could be present in solid Y (1mk)
- Table III (2mks)
QUESTION 3 (9 MARKS)
You are provided with solid F.
Procedure
Carry out the tests below using solid F. write the observations and inferences in the spaces provided.
- Place all solid F in a dry boiling tube. Add about 15cm3 of distilled water and shake thoroughly. Use 2cm3 portions of the solution for tests (b) to (e) below.
Observations Inference (1mk) (1mk) - To the first portion add two drops of universal indicator and record the color and PH.
Observations Inference (1mk) (1mk) - To the second portion add a spatula and full of sodium carbonate.
Observations Inference (1mk) (1mk) - To the third portion add two drops of bromine water.
Observations Inference (1mk) (1mk) - To the fourth add three drops of acidified potassium manganate (VII)
Observations Inference (1mk) (1mk)

MARKING SCHEME
QUESTION 1
Procedure 1
- Tables I and II
- Complete table
- Decimals
- Accuracy
Each table is 3marks
- Average volume for table 1 is about 12.5cm3.
-
- Moles of solution N is 25cm3
= 0.02 × 25 = 0.0005
1000 - Mole ratio 1:5
Moles of H = 1/5 × 0.0005 = 0.0001 - Concentration of H in moles per litre
0.0001 × 1000
5Av.vol
10.00001 × 1000
12.5
= 0.008M
- Moles of solution N is 25cm3
Procedure II
- Average volume for table II is about 18.2cm3
-
- Number of moles of solution H used
ans in =
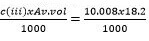
=0.0001456 - Moles of × in 25cm3 solution
Mole ration H:X = 2:5
=5/2 × ans in (f) (i)
= 5/2 × 0.0001456
= 0.000364 - Concentration of x in moles per litre
= Ans in (f) (ii) × 1000
25
= 0.000364 × 100025
25
=0.01456
- Number of moles of solution H used
Question 2a (1mk each)
| Observations | Inference | |
| a | White solid dissolves to form a colourless solution | Cu2+, Fe2+, Fe3+ absent |
| i | White ppt, soluble in excess | Zn2+, Pb2+ or Al3+ present |
| ii | white ppt insoluble in excess | Pb2+ or Al3+ present Zn2+ absent |
| iii | No white ppt | Al3+ present Pb2+ absent |
Question 2b (½mk each)
Solid Color of Flame Sodium chloride golden yellow potassium chloride blue/purple/lilac calcium chloride red y blue/purple/lilac - Cation present in Y is K+.
Question 3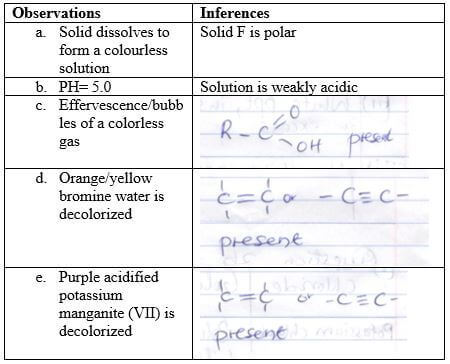

CONFIDENTIAL 2021
Each candidate will require the following
- About 150cm3 solution H
- About 100cm3 solution X
- About 100cm3 solution N
- About 40cm3 1M sulphuric (VI) acid
- About 1.0g solid Y
- About 1.2g solid F
- One 10ml measuring cylinder
- One burette
- One 25ml pipette and pipette filler
- Two conical flasks
- Two boiling tubes
- Four test tubes
- Glass rod
- Distilled water
Candidates should have access to the following
- 2M potassium chloride solution
- 2M calcium chloride solution
- 2M Sodium hydroxide solution
- 2M sodium chloride solution
- 2M ammonia solution
- Bromine water
- Acidified potassium manganate (VII) solution
- Universal indicator solution
- PH chart
- Burner
NB: Supply solution 3, 4, 5, 6, 7 and 8 with droppers.
NOTES- Solution H is prepared by weighing accurately 1.3g of potassium manganite (VII), dissolve in about 400cm3 of distilled water and make the solution to 1 litre. The solution should be prepared one day before the practical is taken.
- Solution N is prepared by dissolving 7.8g of ammonium iron (II) sulphate in 400cm3 of 1M sulphuric (VI) acid and diluting the solution with distilled water to make 1 litre solution.
- Solution X is prepared by measuring accurately 5cm3 of fresh sample of 20 volume hydrogen peroxide, then dilute it with distilled water to make one litre solution.
- 1M sulphuric (VI0 acid is prepared by measuring accurately 55cm3 of concentrated sulphuric (VI) acid, pour carefully into a beaker containing 400cm3 of distilled water then top up with more distilled water to make 1 litre solution.
- Solid Y is prepared by mixing equal amounts of aluminium chloride and potassium chloride thoroughly.
- Solid F is maleic acid.
Download Chemistry Paper 3 Questions and Answers - Form 3 Term 3 Opener Exams 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

