CHEMISTRY
PAPER 1
TERM 2 OPENER EXAM
INSTRUCTIONS
- Answer all the questions
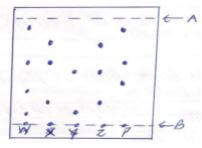
- Dyes W,X, Y and Z were analysed using P as the purest sample of the dye required by a chemist .the result obtained were as shown on the chromatogram below.
- What names are given to the lines marked?
- A 1mk
- B 1mk
- State with a reason the most unsuitable dye for the chemist to use . 2mks
- What names are given to the lines marked?
-
- Distinguish between allotropes and isotopes. 2mks
- Name the two allotropes of sulphur . 2mk
- The table below shows the atomic numbers of elements F G and H.
Which of the elements is the least reactive? Explain 2mksElement F G H Atomic
Number4 12 20 - Classify the changes below as physical or chemical changes.
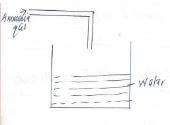
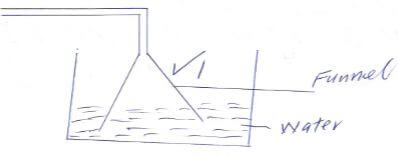
Type of charge Magnetization of iron Heating iron until it softens Souring of milk - A student prepared ammonia gas and wanted to make it solution in water. Complete the diagram to show how he made the solution.
- Element J belong to period 3 and group V.
- Write the electron arrangement of J. 1mk
- The equation of the reaction when J reacts with oxygen gas. 2mks
- A fixed mass of a gas has a volume of 200cm3 at a temperature of 47oC and 750mmHg .calculate the volume the gas would occupy at 7oC and 750mmHg pressure. 3mks
- The diagram below represents a luminous flame.
Mark and label on the diagram the hottest and coolest parts 2mks - Burning magnesium was lowered in a gas jar full of dry sulphur IV oxide.
- State the observation that was made. 2mks
- Write the equation for the reaction occurring. 1mk
- State the reducing agent in the above reaction. 1mk
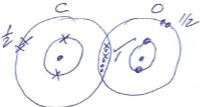
- Draw a dot (.) and cross (X) diagram to show the bonding in the molecules of :
(Atomic number of carbon is 6, Oxygen is 8 and hydrogen is 1)- Carbon II oxide
- Water.
- Explain the following observation
- Magnesium is a better electrical conductor than sodium. 1mk
- A solution of aluminum chloride has PH of 3.0. 1mk
- A compound W consist of 26.7%carbon, 2.2%hydroen and the rest oxygen. It has a relative formula Mass of 90(C=12, H=1, O=16,)
- Determine it empirical formula. 2mks
- Workout its molecular formula 2mks
- Hydrogen was passed over copper II oxide in a combustion tube.
- Write an equation for the reaction that took place. 1mk
- What observation was made in the combustion tube? 2mks
- Name two other gases which could be used to reduce copper II oxide. 2mks
-
- State grahams law of diffusion. 1mk
- 20cm3 of oxygen diffused through a porous partition in 70s .how long would it take 20cm3. Of sulphur VI oxide to diffuse through the same portion under the same condition. 3mks
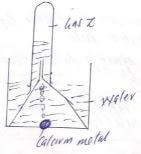
- He set up below was used to collect gas Z produced by the reaction between water and calcium metal.
- Give a chemical test for gas Z. 2mks
- Write the equation of the reaction between the calcium metal and water. 2mks
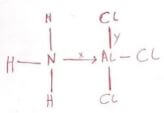
- The diagram below shoes the bonding between aluminium chloride and ammonia
- Name the type of bond labeled
- X. 1mk
- Y 1mk
- How many electrons are used for bonding in the molecule? 1mk
- Name the type of bond labeled
- Graphite is a non metal, yet it conduct an electric current .explain this observation. 1mk
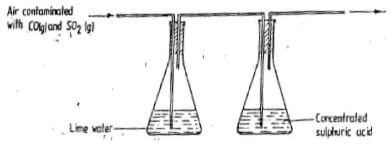
- A sample of air contaminated with carbon monoxide and sulphur dioxide was passed through the apparatus shown in the diagram below.
Which contaminant was removed by passing the contaminated air through the apparatus?
Explain. (2mks) - Explain how you would show chemically that a given gas is ammonia. 2mks
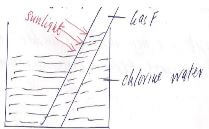
- Chlorine water was exposed to sunlight for a few hours .Colourless gas F was collected in the boiling tube as shown.
- Name gas F. 1mk
- Write an equation for the formation of gas F.2mks
- The equation below shows the action of heat on three metal nitrates.
2S(NO3)2 → 2SO + 4NO2 +O2
2TNO3 → 2TNO2 + O2
2UNO3 → 2U +2NO2 + O2- Arrange the metals S,T and U in the order of reactivity from least to the most reactive. 3mks
- Give one element that could be used. 1mk
-
- Define the term transition temperature concerning the allotropes of sulphur. 1mk
- Draw the structure of a sulphur molecule. 1mk
- Dilute hydrochloric acid was added to a compound Z of sodium. The solid reacted with the acid to form a colorless solution H and a colourless gas K which formed a white precipitate when bubbled through lime water.
- Write the formula of:
- Compound Z 1m
- Colorless gas K 1mk
- Write an equation for the reaction that took place. 2mks
- Write the formula of:
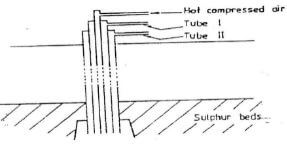
- The diagram below represents the extraction of sulphur by Frasch process
- Name the substance that passes through tube;
I ½ mk
II ½ mk - What is the purpose of hot compressed air in this process? (1mk)
- Name the substance that passes through tube;
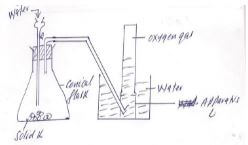
- A student used the setup below to prepare oxygen gas in the laboratory. Study it and answer the questions that follow.
- Identify solid K. 1m
- Name the apparatus labeled L. 1mk
- Write an equation for the reaction in the conical flask. 2mk
- Nitrogen gas is used as an inert atmosphere in electric lamps.
- Explain in terms of structure and bonding why nitrogen gas is so stable .2mks
- Give a reason why despite this stability nitrogen reacts with burning magnesium metal. 1mk
MARKING SCHEME
- Dyes W,X, Y and Z were analysed using P as the purest sample of the dye required by a chemist .the result obtained were as shown on the chromatogram below.
- What names are given to the lines marked?
- A - Solvent front
- B - Baseline
- State with a reason the most unsuitable dye for the chemist to use . 2mks
- Z is the most impure with 3 substances not in the pure dye
- What names are given to the lines marked?
-
- Distinguish between allotropes and isotopes. 2mks
- Allotropes are two or more forms of an element in the same physical state
- Isotopes are atoms of the same element having the same atomic number but different mass numbers
- Name the two allotropes of sulphur . 2mk
- Rhombi sulphur
- monoclinic sulphur
- Distinguish between allotropes and isotopes. 2mks
- The table below shows the atomic numbers of elements F G and H.
Which of the elements is the least reactive? Explain 2mksElement F G H Atomic
Number4 12 20 - F- Has the smallest atomic radius/size and has the least tendency to lose electrons
- F- Has the smallest atomic radius/size and has the least tendency to lose electrons
- Classify the changes below as physical or chemical changes.
Type of change Magnetization of iron Physical Heating iron until it softens Physical Souring of milk Chemical - A student prepared ammonia gas and wanted to make it solution in water. Complete the diagram to show how he made the solution.
- Element J belong to period 3 and group V.
- Write the electron arrangement of J. 1mk
- 2.8.5
- The equation of the reaction when J reacts with oxygen gas. 2mks
- 4J(s) + 3O2(g) → 2J2O5(g)
- 4J(s) + 3O2(g) → 2J2O5(g)
- Write the electron arrangement of J. 1mk
- A fixed mass of a gas has a volume of 200cm3 at a temperature of 47oC and 750mmHg .calculate the volume the gas would occupy at 7oC and 750mmHg pressure. 3mks
- v1=200cm3
T1=47º=47+273=320k
P1=750mmhg
T2=7º=7+273=280k
P2=750mHg
V1/T1=V2/T2
V2=V1T2/T1=200 x 280/320=175cm3
- v1=200cm3
- The diagram below represents a luminous flame.
Mark and label on the diagram the hottest and coolest parts 2mks - Burning magnesium was lowered in a gas jar full of dry sulphur IV oxide.
- State the observation that was made. 2mks
- White ash and yellow specks formed
- Write the equation for the reaction occurring. 1mk
- 2Mg(s) + SO2(g) → 2Mg(s) + S(s)
- 2Mg(s) + SO2(g) → 2Mg(s) + S(s)
- State the reducing agent in the above reaction. 1mk
- Magnesium
- Magnesium
- State the observation that was made. 2mks
- Draw a dot (.) and cross (X) diagram to show the bonding in the molecules of :
(Atomic number of carbon is 6, Oxygen is 8 and hydrogen is 1)- Carbon II oxide
- Water.
- Carbon II oxide
- Explain the following observation
- Magnesium is a better electrical conductor than sodium. 1mk
- Magnesium has 2 delocalised electrons compared to sodium which has 1
- Magnesium has 2 delocalised electrons compared to sodium which has 1
- A solution of aluminum chloride has PH of 3.0. 1mk
- Aluminium chloride hydrolyses in solution to form an acidic solution
- Aluminium chloride hydrolyses in solution to form an acidic solution
- Magnesium is a better electrical conductor than sodium. 1mk
- A compound W consist of 26.7%carbon, 2.2%hydroen and the rest oxygen. It has a relative formula Mass of 90(C=12, H=1, O=16,)
- Determine it empirical formula. 2mks
- %age of oxygen= 100-(26.7+2.2)=71.1%
C H O % composition 26.7 2.2 71.1 RAM 12 1 16 No. of molecules 26.7/12 2.2/1 71.1/16 2.2 2.2 4.4 2.2/2.1 2.2/2.2 4.4/2.2 1 1 2
- %age of oxygen= 100-(26.7+2.2)=71.1%
- Workout its molecular formula 2mks
(CHO2)n=90
12n+n+32n=90
n=2
MF is C2H2O4
- Determine it empirical formula. 2mks
- Hydrogen was passed over copper II oxide in a combustion tube.
- Write an equation for the reaction that took place. 1mk
H2(g) + CuO(s) → H2O(l) + Cu(s) - What observation was made in the combustion tube? 2mks
- Black copper(ii) oxide turned brown
- Name two other gases which could be used to reduce copper II oxide. 2mks
- Ammonia gas and carbon(ii)oxide gas
- Write an equation for the reaction that took place. 1mk
-
- State grahams law of diffusion. 1mk
- Graham's law of diffusion states that under the same conditions of temperature and pressure, the rate of diffusion of a gas is inversely porporional to the sqaure root of its density
- Graham's law of diffusion states that under the same conditions of temperature and pressure, the rate of diffusion of a gas is inversely porporional to the sqaure root of its density
- 20cm3 of oxygen diffused through a porous partition in 70s .how long would it take 20cm3. Of sulphur VI oxide to diffuse through the same portion under the same condition. 3mks
- MO2=2 x 16=32
MsO3=(32+16x3)=80
TO2=70secs
TsO3=?
TsO3=√MsO3
TO2 √MO2
TsO3= 70√80/√32 - -70 x 8.944/5.657=110secs
- MO2=2 x 16=32
- State grahams law of diffusion. 1mk
- He set up below was used to collect gas Z produced by the reaction between water and calcium metal.
- Give a chemical test for gas Z. 2mks
- It burns with a pop sound
- Write the equation of the reaction between the calcium metal and water. 2mks
- Ca(s) + 2H2O(l) → Ca(OH)2(aq)+H2(g)
- Ca(s) + 2H2O(l) → Ca(OH)2(aq)+H2(g)
- Give a chemical test for gas Z. 2mks
- The diagram below shoes the bonding between aluminium chloride and ammonia
- Name the type of bond labeled
- X.- co-ordinate/dative bond
- Y - covalent bond
- How many electrons are used for bonding in the molecule? 1mk
- 14 electron
- 14 electron
- Name the type of bond labeled
- Graphite is a non metal, yet it conduct an electric current .explain this observation. 1mk
- Graphite has delocalised electrons in its structure.
- Graphite has delocalised electrons in its structure.
- A sample of air contaminated with carbon monoxide and sulphur dioxide was passed through the apparatus shown in the diagram below.
Which contaminant was removed by passing the contaminated air through the apparatus?
Explain. (2mks)- Sulphur(iv)oxide
Carbon(ii)oxide gas does not react with lime/ lime water nor conc H2SO4 but SO2 being acidic is absorbed by the lime water.
- Sulphur(iv)oxide
- Explain how you would show chemically that a given gas is ammonia. 2mks
- Dip a glass rod in concentrated hydrochloric acid and introduce if near the unknown gas if denser white fumes are formed, then the gas is ammonia
OR - if turns damp red litmus paper blue, it is ammonia.
- Dip a glass rod in concentrated hydrochloric acid and introduce if near the unknown gas if denser white fumes are formed, then the gas is ammonia
- Chlorine water was exposed to sunlight for a few hours .Colourless gas F was collected in the boiling tube as shown.
- Name gas F. 1mk
- Gas F- Oxygen gas
- Write an equation for the formation of gas F.2mks
- 2HOCl(aq) →2HCl(aq) + O2(g)
- Name gas F. 1mk
- The equation below shows the action of heat on three metal nitrates.
2S(NO3)2 → 2SO + 4NO2 +O2
2TNO3 → 2TNO2 + O2
2UNO3 → 2U +2NO2 + O2- Arrange the metals S,T and U in the order of reactivity from least to the most reactive. 3mks
U → S → T
least →most - Give one element that could be used. 1mk
- silver or mercury
- Arrange the metals S,T and U in the order of reactivity from least to the most reactive. 3mks
-
- Define the term transition temperature concerning the allotropes of sulphur. 1mk
- Transition temperature is the temperature at which one allotrope of sulphur changes to another
- Draw the structure of a sulphur molecule. 1mk
- Define the term transition temperature concerning the allotropes of sulphur. 1mk
- Dilute hydrochloric acid was added to a compound Z of sodium. The solid reacted with the acid to form a colorless solution H and a colourless gas K which formed a white precipitate when bubbled through lime water.
- Write the formula of:
- Compound Z 1m
- Na2CO3
- Colorless gas K 1mk
- CO2
- Write an equation for the reaction that took place. 2mks
- Na2CO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l)+CO2(q)
- Compound Z 1m
- Write the formula of:
- The diagram below represents the extraction of sulphur by Frasch process
- Name the substance that passes through tube;
I - Molten sulphur
II - superheated water - What is the purpose of hot compressed air in this process? (1mk)
- To force out molten sulphur from underground.
- Name the substance that passes through tube;
- A student used the setup below to prepare oxygen gas in the laboratory. Study it and answer the questions that follow.
- Identify solid K. 1m
- Sodium peroxide
- Name the apparatus labeled L. 1mk
- Beehive stand
- Write an equation for the reaction in the conical flask. 2mk
- 2Na2O2(s) + 2H2(l) → 4NaOH(aq) + O2(g)
- 2Na2O2(s) + 2H2(l) → 4NaOH(aq) + O2(g)
- Identify solid K. 1m
- Nitrogen gas is used as an inert atmosphere in electric lamps.
- Explain in terms of structure and bonding why nitrogen gas is so stable .2mks
- Atoms of nitrogen are bonded by three strong covalent bonds which need a lot of energy to break
- Give a reason why despite this stability nitrogen reacts with burning magnesium metal. 1mk
- Burning magnesium releases a lot of heat energy which gets absorbed by the nitrogen molecule breaking the triple covalent bond.
- Explain in terms of structure and bonding why nitrogen gas is so stable .2mks
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions And Answers - Form 3 Term 2 Opener Exams 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students