CHEMISTRY
PAPER 2
TERM 2 OPENER EXAM
INSTRUCTIONS
- Answer all the questions
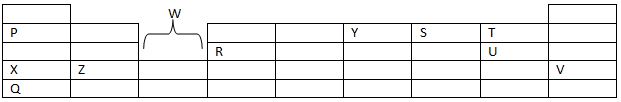
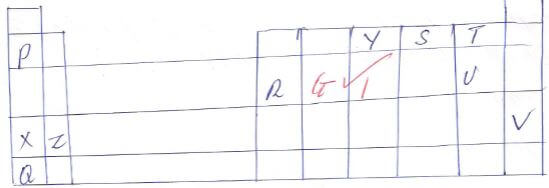
- The grid below shows part of the table. The letter is not the actual chemical symbols of the elements. Study it and use it to answer the question that follows.
- Which element is the:
- Most reactive non metal. 1mk
- Most reactive metal. 1mk
- What name is given to element that belongs to the regions marked W.? 1mk
- What type of bond if formed in the compound when element Z reacts with element U? Give a reason for your answer. 2mks
- Compare the atomic radius of:
- T and U. 2mks
- Z and X. 2mks
- Write the formula of the compound formed when:
- Element Z reacts with element Y 1mk
- Element P reacts with element V. 1mk
- Given that element G has an electron arrangement of 2:8:4 put it in it correct position on the above grid. 1mk
- Which element is the:
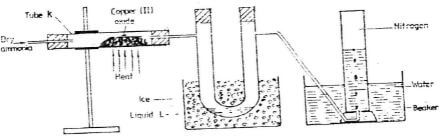
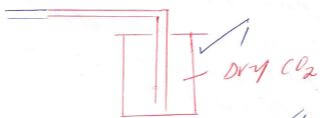
- The diagram below shows a set-up that can be used to obtain nitrogen gas in an experiment.
- Name liquid L (1mk)
- What observation would be made in tube K after heating for some time? (1mk
- Write an equation for the reaction that took place in tube K. (1 mk)
- If 320 cm3 of ammonia gas reacted completely with the copper?
Calculate:- Volume of nitrogen gas produced. (1mk)
- the mass of copper oxide that reacted (3mks)
(Cu = 63.5, O=16.O, one mole of gas occupies 24 liters at room temperature and pressure)
- At the end of experiment the PH of the water in the beaker was found to be about 11. Explain (2mks)
- In another experiment a gas jar containing ammonia was inverted over a burning splint. What observation would be made? (1mk)
- Why is it advisable to obtain nitrogen from air instead ammonia? (1mk)
- The diagram below shows a set-up that can be used to obtain nitrogen gas in an experiment.
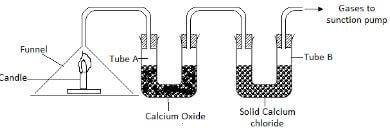
- A form three student from Anestar High School used the set up drawn below in an attempt to prepare dry carbon IV oxide .Study it carefully and use it to answer the questions that follows.
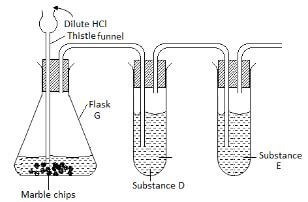
- Identify two faults in the set up of the apparatus. 2mks
- Complete the diagram to show how dry carbon IV oxide gas can be collected. 1mk
- Name substances D and E and give their purposes in the above set up
- Substance D. 1mk
- Purpose 1mk
- Substance E. 1mk
- Purpose 1mk
- Write the equation of the reaction in the flask G. 2mk
- State and explain the observation made when dilute sulphuric acid is used in place of hydrochloric acid. 2mks
- Give any two uses of carbon IV oxide gas. 2mks
- The scheme below shows various reactions starting with hydrogen and nitrogen .study it carefully and answer the question that follows.
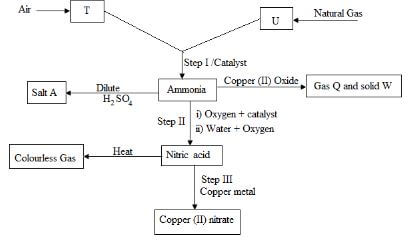
- Name the substances
- T 1mk
- U 1mk
- A 1mk
- Q 1mk
- W 1mk
- C 1mk
- Name the catalyst which could be used in:
- Step I 1mk
- Step II 1mk
- Write equations for the reaction occurring in:
- Step I 1mk
- Step II 2mks (two equations)
- What property of ammonia gas is shown in its reaction with copper II oxide? 1mk
- Give one industrial use for:
- Ammonia 1m
- Nitric acid 1mk
- Name the substances
-
- Candle wax is mainly a compound consisting of two elements. Name the two elements. 2mks
- The set up below was used to investigate the burning of a candle study it and answer the questions that follow.
- What would happen to the burning candle if the pump was turned off? Give a reason 1mk
- State and explain the changes in mass that is likely to occur in tube A by the end of the experiment. 3mks
- Name two gases that come out through tube C. 2mks
- Write two equations for the reactions taking place when a candle burns. 4mks
-
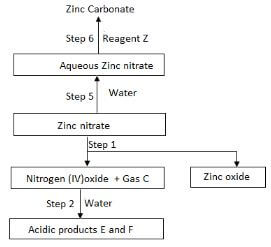
- The flow charts below shows some reaction starting with zinc nitrate. Study it and answer the question that follows.
- State the condition necessary in step I. 1mk
- Identify:
- Reagent Z 1mk
- Gas C 1mk
- Acidic product E and F. 2mks
- Write equation for the reaction in:
- Step I 1mk
- Step II 1mk
- Step VI 1mk
- The flow charts below shows some reaction starting with zinc nitrate. Study it and answer the question that follows.
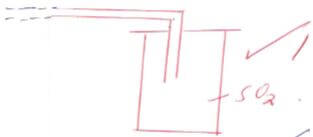
- The diagram below shows the set up used by a student to prepare dry sulphur IV oxide gas. Study it carefully and answer the question that follows
- Complete the diagram to show how to collect dry sulphur IV oxide. 1mk
- Write an equation for the reaction in the flask. 2mk
- Give another pair of chemical that could be used to prepare sulphur IV oxide. 2mks
- Distinguish between the bleaching action of sulphur IV oxide and chlorine. 2mks
- Sulphur IV oxide gas was bubled through concentrated nitric V acid .State and explains the observations made. 2mks
MARKING SCHEME
-
-
- T
- Q
- Transition elements
- ionic/ electrovalent bond. There is complete transfer of electrons from Z to U
-
- U has more occupied energy levels than T hence has a larger atomic radius than T
- Z has a smaller atomic radius than X. Z has more protons and therefore a higher nuclear charge which aattracts its electrons more strongly.
-
- Z3Y2
- NO compound formed as is stable
-
-
-
- Liquid L - water
- Black copper(ii) oxide changes to brown copper metal
- 3CuO(s) + 2NH3(g) → 3Cu(s) + 3H2O(l) + N2(g)
-
- 2 x 24 litres of NH3 produce 24 litres N2.
4800cm3 produce 24000 cm3 N2
320cm3 NH3 produce 320/48000 x 238.5g CuO=1.59g - 2moles NH3 produce 3 moles CuO
48000cm3 NH3 produce 238.5g CuO
320cm3 NH3 produce 320/48000 x 238.5g CuO
- 2 x 24 litres of NH3 produce 24 litres N2.
- Ammonia dissolved in the water to form ammonia solution which is a weak base
- The splint is extinguish as ammonia does not suport burning
- Air has a plentidul supply of nitrogen
-
-
- Thirstle funnel has not reached the bottom of the flask G. Delivery tube leading the gas into substance should dip in it.
-
- D- water- removes hydrogen vhloride
E- conc sulphuric acid- dries the carbon(iv) oxide gas. - CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
- The reaction would start but stop immediately. This is due to formation of insoluble calcium sulphate which coats the unreacted carbonate preventing further reaction
-
- Used in fire extinguishers
- Used as a refrigerating agent for perishable gods
- Manufacture of sodium carbonate in the solvay process
- Used in making aerated drinks.
-
-
-
- T- Nitrogen
- U- Hydrogen
- A-ammonium sulphate
- Q-nitrogen gas
- W-copper
- C-oxygen gas
-
- Step I- iron
- Step II- platinum/ rhodium
-
- Step I: N2(g) + 3H2(g) ⇌ 2NH3(g)
- Step II: 4NH3(g)+5O2(g) → 4NO(g)+6H2O(l)
4NO2(g) + O2(g) + 2H2O(l) → 4HNO3(aq)
- It is a reducing agent
-
- Amonia
- As a fertilizer
- Manufacture of nitrogenous fertilizer
- As a refrigerant
- Softening hard water
- Removal of greasy stains
- Manufacture of hydrazine that is used as rocket fuel
- Nitric acid
- Manufacture of fettilizers
- Manufacture of explosives
- Manufacture of dyes and drugs
- Purification of metals such as silver and gold
- Etching designs on some metals
- Amonia
-
-
-
- Carbon and hydrogen
-
- It would be extinguished. this is due to build up of carbon(iv)oxide in the funnel around the burning candle
- Mass increases calcium oxide absorbs water to form calcium hydroxide then absorbs carbon (iv) oxide gas.
-
- Argon
- Nitrogen
- C(s) + O2(g) → CO2(g)
2H2(g) + O2(g) → 2H2O(l)
-
-
- Heating
-
- Reagent Z - Na2CO3, K2CO3 or (NH4)2CO3
- Gas C- oxygen gas
- E- nitric (ii) acid
F-nitric (v) acid
-
- 2 Zn(NO3)2(s) → 2ZnO(s)+4NO2(g)+O2(g)
- 2NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq)
- Zn(NO3)2(aq) + Na2CO3(aq) → ZnCO(l) + 2NaNO3
Use of Na2CO3 or K2CO3 or
(NH4)2CO3 in the above equation.
-
-
-
- Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + SO2(g)
- Copper and conc sulphuric acid
- SO2 bleaches by reduction hence temporary Cl2 bleaches by oxidation hence permanent.
- Brown fumes of NO2 are produced. SO2 reduces conc HNO3 forming brown fumes of NO2
-
Download Chemistry Paper 2 Questions And Answers - Form 3 Term 2 Opener Exams 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students