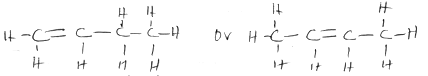INSTRUCTIONS TO CANDIDATES
- Answer all the questions in the spaces provided in the question paper.
- Mathematical tables and silent electronic calculators may be used.
- All working must be clearly shown where necessary.
- Elements burn in oxygen to form basic or acidic oxides. Name two elements which form acidic oxides. (2 mks)
- Element T has atomic number 9 while V has atomic number 11.
- Write down the electronic configurations of elements T and V.
T ……………………….. (½mk)
V ……………………..... (½ mk) - State the type of bond formed when T and V combine. (1 mk)
- Write down the electronic configurations of elements T and V.
- P, Q, R and S are metals. P reacts with steam whereas Q is not affected by either cold water or steam. R reacts violently with cold water while S bursts into flames as soon as it comes into contact with cold water. Arrange the metals in order of decreasing reactivity.(2 mks)
- When 27.8g of hydrated aluminium oxide (Al2O3.XH2O) was heated to a constant mass, 20.6g of aluminium oxide was obtained. Determine the value of x (H=1, O=16, Al=27).
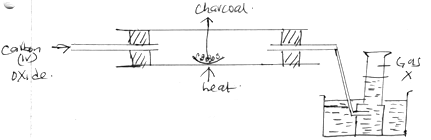
(3 mks) - The following diagram shows carbon(iv)oxide passed over heated charcoal to produce gas X.
- Identify gas X. (1 mk)
- Write an equation for the reaction which produces gas X. (1 mk)
- The above experiment should be carried out in a fume chamber. Why? (1 mk)
- When a piece of sodium metal was put in a beaker of water, it darted on the surface before dissolving.
- Write an equation for the reaction between sodium metal and water. (1 mk)
- What is the effect of the solution formed in (a) above on red and blue litmus papers? Explain. (2 mks)
- 22.2 cm3 of sodium hydroxide solution containing 4.0g per litre of sodium hydroxide were required for complete neutralization of 0.1g of a dibasic acid. Calculate the relative formula mass of the dibasic acid. (Na=23.0, O=16.0, H=1.0). (3 mks)
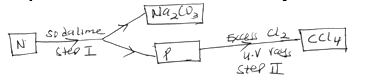
- Study the flow chart below and answer the questions that follow.
- Identify N and P. (2 mks)
N ……….
P ……….. - What name is given to the reaction in step II? (1 mk)
- Identify N and P. (2 mks)
- Molten Lead(ii)Bromide was electrolysed using graphite electrodes. Write half equations for the reaction occurring at each electrode. (2 mks)
Cathode:
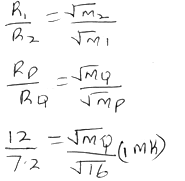
Anode: - Gas P diffuses through a porous material at a rate of 12cm3s-1, whereas gas Q diffuses through the same material at a rate of 7.2cm3s-1. Given that the molar mass of P is 16, calculate the molar mass of Q. (3 mks)
- Element R has an atomic number of 6 and s has an atomic number of 9. Using dot (.) and cross(x) diagram show how R and S combine to form a compound. (2 mks)
- The table below shows the Ph values of solutions I, II, III, and IV.
Solution I II III IV PH 2 7 11 14 - Which solution is likely to be sodium chloride solution. (1 mk)
- A few drops of phenolphthalein indicator were added to solution (iv). State and explain the observations made. (2 mks)
- The molecular formula of a hydrocarbon is C6H14. The hydrocarbon can be converted into two other hydrocarbons as shown by the equation below.
C6H14 → C2H6 + X- Name and draw the possible structural formula of X. (2 mks)
- State the observation that would be made if a few drops of acidified potassium manganate(vii) were added to a sample of X. (1 mk)
- When magnesium is heated in air, it forms a solid Q and solid P. when solid Q is reacted with water it produces a gas W that turns moist red litmus paper to blue. Identify;
- Solid Q …………… (1 mk)
- Solid P ……………. (1 mk)
- Write an equation for the formation of gas W. (1 mk)
- The empirical formula of hydrocarbon is C2H3. The hydrocarbon has a relative molecular mass of 54. (H=1.0, C=12.0).
- Determine the molecular formula of the hydrocarbon. (1 mk)
- Draw the structural formula of the hydrocarbon. (1 mk)
- To which homologous series does the hydrocarbon drawn in (b) above belong?
(1 mk)
- Give the name of each of the processes described below which takes place when salts are exposed to the air for sometime.
- Anhydrous copper(ii)sulphate becomes wet. (1 mk)
- Magnesium chloride forms an aqueous solution. (1 mk)
- Fresh crystals of sodium carbonate become covered with white powder. (1 mk)
- A gas occupies 4dm3 at a pressure of 152 mmHg. Calculate the gas pressure when the volume is reduced to 1.5dm3. (2 mks)
- When a white powder P was heated it decreased in mass and produced solid X which was reddish brown when hot and yellow when cold. A gas R which formed a white precipitate with calcium hydroxide was also evolved.
- Identify substances P and X.
P …………………… (1 mk)
X …………………… (1 mk) - Write an equation for the formation of the white precipitate. (1 mk)
- Identify substances P and X.
- Starting with lead(ii)carbonate explain how you would prepare a pure sample of lead(ii)chloride. (3 mks)
- Study the information in the table below and answer the questions that follow.
Element Atomic radius(nm) Ionic radius (nm) W 0.144 0.195 X 0.072 0.136 Y 0.133 0.216 Z 0.099 0.181 - Are the above elements metals or non metals? Explain. (2 mks)
- Select the most reactive element in the table above. Explain. (1 mk)
-
- Explain why the metals magnesium and aluminium are good conductors of electricity. (1 mk)
- Other than cost, give two reasons why aluminium is used for making electric cables while magnesium is not. (2 mks)
- Determine the volume of hydrogen gas formed when excess zinc metal is added to 1100cm3 of 1m hydrochloric acid. (1 mole of gas occupies 24.0 litres at room temperature). (2 mks)
- Metal P is a group II element in the periodic table and it lies below Q in the same group.
- Explain how the reactivity of metal P and Q with bromine compare. (1 mk)
- Given that the atomic number of Q is 12, determine the atomic number of P. show how you arrive at your answer. (2 mks)
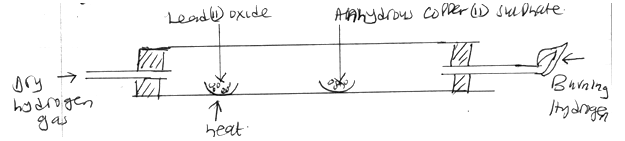
- Study the diagram below and answer the questions that follow.
- What is observed on the anhydrous copper(ii)sulphate? (1 mk)
- Write an equation for the reaction between lead(ii)oxide and hydrogen. (1 mk)
- State another observation apart from that one in (a) made in the combustion tube.(1 mk)
- Iron roofs usually turn brown after some time as a result of formation of rust on their surfaces.
- Explain whether rusting is a physical or a chemical change. (2 mks)
- State one way of preventing rusting. (1 mk)
- A student reacted lead(ii)carbonate with sulphuric(vi)acid in order to prepare lead(ii)sulphate salt.
- Explain why he was unable to prepare the lead(ii)sulphate salt using the above reagents. (2 mks)
- Give another acid he would use in place of sulphuric (vi) acid. (1 mk)
- In a reaction to prepare ammonia gas 15 litres of hydrogen gas was reacted with 10 litres of nitrogen gas.
- Determine the volume of the gas that was not completely used in the reaction.
(2 mks) - Calculate the volume of ammonia gas produced in the reaction. (1 mk)
- Determine the volume of the gas that was not completely used in the reaction.
- When sodium nitrate is heated, it produces sodium nitrite and gas C.
- Identify gas C. (1 mk)
- Name the type of reaction undergone by the sodium nitrate. (1 mk)
- When an electric current was passed through two molten substances E and F in separate electrolytic cells. The observations recorded below were made.
Complete the table above. (2 mks)Substance Observation Type of structure E Conducts electric current and a gas is formed at one of the electrodes. F Conducts an electric current and is not decomposed. - State two use of nitrogen gas. (2 mks)

MARKING SCHEME
-
- Sulphur
- Phosphorus
- Carbon
- Nitrogen
Any two 1mk each
-
- T-2.7
V-2.8.1 - Ionic bonding
- T-2.7
- S→R→P→Q
decreasing reactivity
All correct - 1mk
Last and First correct 1m
Al2O3 : H2OAl2O3 H2O Mass
R.F.M
No. of moles
Divide by the smallest20.6
102
20.6
102
0.2019
0.2019
0.20197.8
18
7.8
18
0.4
0.4
0.2019
1 : 1.98
1 : 2
x=2-
- Carbon (ii) Oxide (1mk)
- CO2(g) + C(s) → 2CO(g) (1mk)
- Carbon (ii) Oxide is poisonous (1mk)
-
- 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) (1mk)
Unblanaced equation - 0mk
wrong/missing state symbols - ½mk penalty - Red litmus turn blue, blue litmus remains blue (1mk) since the solution formed (NaOH) is alkaline (1mk)
- 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) (1mk)
- 2NaOH(aq) + H2X(aq) → Na2X(aq) + 2H2O(l)
2 : 1 ½mk
Molarity of NaOH = 4/40 = 0.1M ½mk
Moles of NaOH = 0.1 × 22.2 = 0.00222moles ½mk
Moles of H2X = ½ × 0.00222 = 0.00111moles ½mk
0.00111moles → 0.1g
1 mole → 0.1 × 1 = 90.00g (1mk)
0.00111 -
- N-Sodium ethanoate (CH3COONa) (1mk)
P-Methane (CH4) (1mk) - Substitution (1mk)
- N-Sodium ethanoate (CH3COONa) (1mk)
- Cathode: Pb2+(aq) + 2e− → Pb(s) (1mk)
Anode: 2Br−(aq) → Br2(g) + 2e−(1mk) -
12 × 4 = 7.2 × √MQ (1mk)
48 = 7.2 × √MQ
√MQ = 48
7.2
√MQ = 6.666
MQ = 44.44g (1mk) - R-2.4
S-2.7
RS4(2mks)
-
- II (1mk)
- The solution turned to pink (1mk) since solution IV is a strong alkali (1mk)
-
- Butene (1mk)
- Purple acidified potassium manganate (VII) is decolourised (1mk) due to the presence of a double bond.
- Butene (1mk)
-
- Solid Q - Magnesium Nitride Mg3N2 (1mk)
- Solid P - Magnesium Oxide MgO (1mk)
- Mg3N2(s) + 6H2O(l) → 3Mg(OH)2(aq) + 2NH3(g) (1mk)
-
- E.f = C2H3
n = 54/27 = 2 (½mk)
M.F = (C2H3) × 2
= C4H6 (1½ mk) -
(1mk)
- Alkynes (1mk)
- E.f = C2H3
-
- Hygroscopy (1mk)
- Deliquescency (1mk)
- Efflorescence (1mk)
- P1V1 = P2V2
4 × 152 = P2 × 1.5 (1mk)
P2 = 4 × 152 = 405.33Hg (1mk)
1.5 -
- P- Lead Carbonate/ PbCO3 (1mk)
X- Lead (II) Oxide /PbO (1mk) - Ca(OH)2(aq) + CO2(g)→ CaCO3(s) + H2O(l) (1mk)
- P- Lead Carbonate/ PbCO3 (1mk)
- React with PbCO3 with dilute nitric (V) acid to form lead (II) Nitrate solution. React lead nitrate solution with a soluble chloride or dilute HCl (1mk) to form lead chloride. Filter out (½mk) lead chloride and dry it (½mk) in between filter papers.
-
- non-metals (1mk) The ionic radius is greater than atomic radius (1mk)
- Z (½mk). It has the smallest atomic radius (½mk) hence can easily gain an electron.
-
- They contain delocalised electrons (1mk)
-
- It is light (1mk @)
- It does not corrode easily (1mk)
- Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
1 : 2 (½mk)
Moles of HCl = 1 × 1100 = 1.1moles (½mk)
1000
Mole ratio HCl:H2
2:1
Moles of H2 = ½ × 1.1 = 0.55moles (½mk)
Volume of H2 = 0.55 × 24L
= 13.2L (½mk) -
- P is more reactive(½mk) with bromine than Q since it has a greater atomic radius (½mk)
- Q=12
P has one more energy level since its below Q. Hence its atomic number
2.8.8.2 =20 (1mk)
-
- White anhydrous Copper (II) Sulphate turns blue (1mk)
- PbO(s) + H2(g) → Pb(s) + H2O(g) (1mk)
- A grey (1mk) solid is observed/ The colour of the solid changes from yellow to grey.
-
- Rusting is a chemical change (1mk) since the reaction is irreversible (1mk)/ Forms a new product.
-
- Electroplating
- Alloying
- Painting
- Oiling and greasing
any one 1mk
-
- PbCO3 reacts with Sulphuric (VI) acid forming insoluble Lead Sulphate (1mk) which hinders further reaction (1mk)
- Nitriv (V) acid (1mk)
- N2(g) + 3H2(g)
2NH3(g)
1vol 3vol 2vol
10L 15L- 15L of H2 requires 15/3L = 5L of N2 (1mk)
hence N2 (1mk) was in excess - Volume of Ammonia gas
1volume =5L
2volumes = 5×2 (½mk)
= 10L (½mk)
- 15L of H2 requires 15/3L = 5L of N2 (1mk)
-
- Oxygen gas (1mk)
- Thermal decomposition (1mk)
-
Substance Observation Type of structure E Conducts electric current and a gas is formed at one of the electrodes. Giant ionic structure (1mk) F Conducts an electric current and is not decomposed. Giant metallic structure (1mk) -
- Storage of semen for artificial insemination.
- Filling empty oil tankers to prevent an explosion.
- Manufacture of Ammonia.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Form 3 Mid Term 2 Exams 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students