Questions
Instructions:
- This paper consists of two sections,EACH section 50 marks.
- All working MUST be clearly shown.
SECTION A
- 60 ml of ozone gas diffused through a porous partition in 5 min 10 sec. how many seconds would it take 80 ml of nitrogen (i) oxide to diffuse through the same partition under same condition. ( N=14, O=16) (3mks)
- Below are 3 isotopes of element Neon. Study it and answer the questions that follows.
Ne- 20 90.9%
Ne – 21 0.3%
Ne – 22 8.8%- What are isotopes (1mk)
- Calculate the relative atomic mass of Neon (3mks)
- 0.28g of Aluminium reacted completely with Oxygen gas. Calculate the volume of oxygen used. [molar gas volume = 24=dm3 Al =27 O = 16] (3mks)
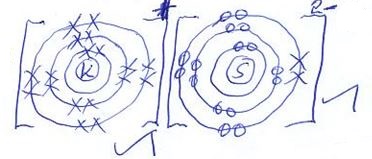
- Using dots (.) and cross(X) diagram, show the bonding in; [C =16 Cl= 17 K = 19 S = 16]
- Potassium Sulphide (11/2 mks)
- Carbon tetrachloride (11/2 mks )
- During laboratory preparatioin of Oxygen, Manganese (iv) Oxide is added to reagent H.
- Name reagent H. (1mk)
- State the role of Manganese (iv) Oxide in this experiment (1mk)
- Write the chemical equation for the reaction that took place (1mk)
- Salt may be classified as soluble or insoluble salt.
- Select from the following list a pair of compounds that can be used to prepare soluble and insoluble salts.
Nitric acid, lead nitrate, Potassium Nitrate, Barium Oxide, Sodium Chloride- Soluble salt (1mk)
- Insoluble salt (1mk)
- Describe how a solid sample of copper (ii) nitrate can be prepared in the lab starting with copper metal (3mk)
- Select from the following list a pair of compounds that can be used to prepare soluble and insoluble salts.
-
- State Charles law (1mk)
- A gas occupies 300ml at a pressure of 570 mmHg and temperature of -136 degrees Celsius. What would be its volume at stp. (3mks)
-
- State Gay Lusacs law. (1mk)
- Under certain condition methane react with steam forming carbon (ii) Oxide and Hydrogen. Calculate the total volume of the of gas that would be form when a 100ml of steam react completely with methane (2mks)
- Ammonia and nitric (v) acid are used to manufacture ammonium nitrate. Calculate the amount of nitric (v) acid required to manufacture 1000kg of ammonium nitrate using excess ammonia gas. [N = 14, H = 1, O = 16] (3mks)
-
- Calculate the number of sodium atomsa present in 40g of sodium metal
[“L” = 6.02 X 1023 Na = 23] (3mks) - Calculate the molarity of sodium hydroxide if 40g of sodium hydroxide was dissolved water to make 500ml of solution. (3mks)
- Calculate the number of sodium atomsa present in 40g of sodium metal
- Describe an experiment to show that group one element reacts with cold water forming an alkaline solution. (3mks)
- Name three methods of gas collection. (3mks)
-
- Candle wax is mainly compound consisting of the elements. Name the two elements (2mks)
- State one industrial use of hydrogen gas (1mk)
-
- Write the chemical formula of rust (1mk)
- List 3 methods of prevent rusting (3mks)
SECTION B
-
- Name two apparatuses that can be used for determining accurate volume in a
laboratory (2marks) - One of the flames produced by Bunsen burner is the luminous flame
- Explain why this flame is very bright (1mark )
- State two disadvantages of the luminous flame (2marks)
- Air is usually one of the substances that is considered as a mixture
- Identify the two most abundant component of air (2marks)
- Give two reasons why the air is considered as a mixture (2marks)
- One of the components of air is carbon (iv) oxide. Describe an experiment that can be used to prove the presence of carbon (iv) oxide in the air (2marks)
- Name two apparatuses that can be used for determining accurate volume in a
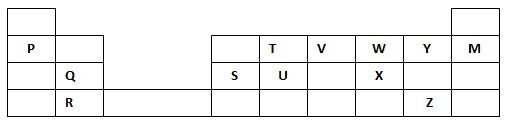
- The grid below forms part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements
- Write the general name given to the element P belong. (1mark)
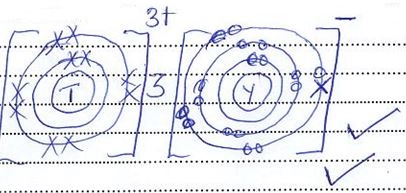
- An element N has an atomic number of 15. Write down its electronic arrangement and hence fix it in its right position on the grid above. (2marks)
Electronic arrangement ………………………………………………………………………… - Compare the size of the atom of R and that of its ion. Explain your answer. (2marks)
- Give the formula of the compound formed between (1mark)
- P and W …………………………………………………………………………
- T and Y …………………………………………………………………………..
- Compare the melting points of element Q and S. Explain (2marks)
- State the unreactive element in the grid. Give a reason for your answer (2marks)
- Give two advantages that element S has over element Q in making electric cables(2mks)
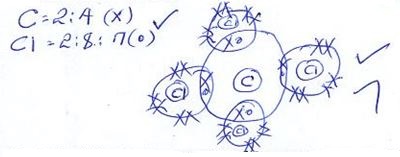
- Draw (a) dot (.) and cross (x) diagram to represent the bonding in compound formed between T and Y(2 marks)
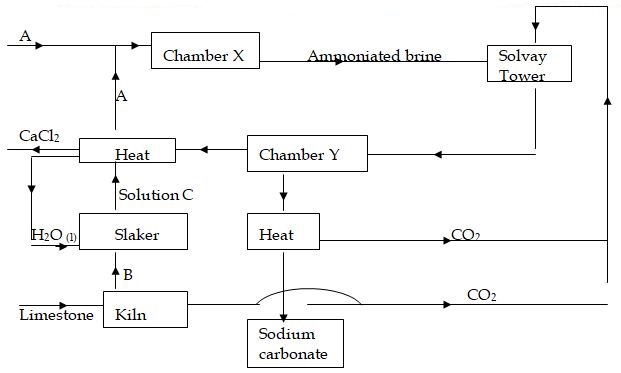
- The chart below represents the main steps in the large-scale manufacture of sodium carbonate.
- Name substances A and B.
- Write down the chemical equation leading to formation of C. (1 mark )
- A stream of cold water is made to circulate around chamber X. What does this suggest about the reaction taking place. (1 mark )
- Name the process that takes place in chamber Y. (1 mark)
- State any 2 by-products recycled in the process. (2 marks)
- state one use of calcium chloride 1mks
- Mention any 2 uses of sodium carbonate. ( 2 mark )
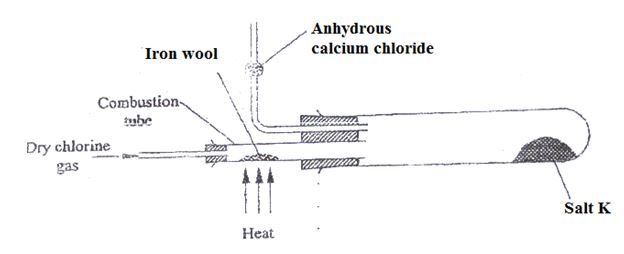
- Study the set up below.
- Name salt K (1mk)
- Write the equation for the reaction for the formation of salt K (1mk)
- What property of salt A is exhibited as shown in the experiment.(1mk)
- What is the purpose of anhydrous calcium chloride? Explain (2mk)
- Name another metal that can be used to produce similar results (1mk)
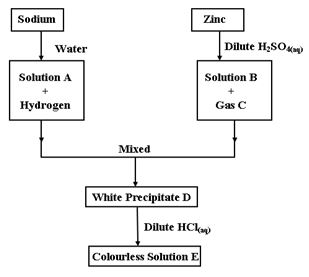
- The scheme below shows the preparation of a certain salt. Study it and answer the questions that follow
- Give the name and the formula of the following (2mk)
Name Formula Solution A Solution B - Give the equation for the;
- Formation of B and gas C (2mk)
- Formation of solution A and hydrogen gas (1mk)
- Describe a chemical test for gas C (2mk)
- Give two observations made when sodium metal is placed on water (2mk)
- Distinguish between anhydrous salt and dry salt (1mk)
- Give the name and the formula of the following (2mk)
Marking Scheme
- 60ml -> 310 sec
80ml -> ?
80 x 310 = 413 sec
60
TN₂O = √N₂O/O3
TO3
TN₂O = TO3(√N₂O/O3)
= 413 x (√(14 x 2 + 16)/16x3)
= 395.4 sec -
- Elements that have some atomic numberr but different mass number
- R.A.M = (20 x 90.9) + (21 x 0.3) + (22 x 8.8)
3
= 20.179
- 4Al(s) + 3O2(g) → 2Al2O3(s)
Moles of Al = 0.28/27 = 0.0104
Moles of O2 (mole rate 4:3)
0.0104 x 3 = 0.0078 moles
4
but moles = Vol/MG
Volume = 0.0078 x 24 = 0.187dm3 -
- Potassium Sulphide
K = 2881
S= 2:8:6
S2-
K+ + S2- →K2S -
- Potassium Sulphide
-
- Hydrogen peroxide
- catalyst// speed up rate of reaction
- 2H2O2(l) → O2(g) + 2H2O(l)
-
-
- BaO & HNO3
- NaCl & Pb(NO3)2
- Heat copper in crucible to obtain CuO
Add excess CuO to warm dilute Nitric (V) acid and stir. Filter. Heat the filtrate until it is saturated.
Allow saturated solution to cool
Place crystals formed in between filter paper
-
-
- Charles’s law - the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant.
-
P1 = 570mmHg
V1 = 300 ml
T1 = -136 + 273= 137K
P2 = 760mmHg
V2 =?
T2 = 273K
P1V1 = P2V2
T1 T2
V2 = P1V1T2
T1P2
V2 = 570 x 300 x 273
137 x 760
= 448.36cm3
-
- states the pressure of a gas varies directly with temperature when mass and volume are kept constant.
- CH4(g) + H2O(g) → CO(g) + 3H2(g)
CO = 100ml
H2 = 100 x 3 = 300ml
- NH3(g) + HNO3(aq) → NH4NO3(aq)
Moles of NH4NO3
1000 = 12.5 moles
(14 x 2 + 1 x 4 + 16 x 3)
Moles of HNO3 - 12.5 moles (mole ratio 1:1)
Mass of HNO3 - 12.5 x (1+14+16x3)
1000 x 1000 = 12
(14x2+1x4+16x3)
Moles of HNO3 - 12 500
Mass of HNO3 - 12 500 x 63 = 787, 500g
≈ 787.5kg -
- Moles of Na - 40/23 = 1.739 moles
But moles
No. of particles
Avagadros No
No of particles = 1.739 x 6.02 x 1023
= 1.0469 x 1024 - Moles of NaOH -
40
23 + 16 + 1
1 mole
molarity = 1000 x 1 = 1M
1000
- Moles of Na - 40/23 = 1.739 moles
- Take a small grain size portion of sodium/potassium//lithium
Place it in a beaker containing a lot of water
Test the resulting solution using red litmus paper it turns blue -
- Over water method
- Downward delivery
- Upward dlivery
-
-
- Hydrogen
- Carbon
-
- Hardening of oil to fat
- in oxy-hydrogen frame to cut metals
- in manufacture of HCL with CL2
-
-
-
- Fe2O3.3H2O
- Greasing/ oiling
- Painting
- electrolysis
- cathode protection
- galvanising
-
-
-
- Volumetric flask
- Pippette
- Burette, measuring cylinder
-
- It has unburnt carbon which is luminous
-
- Its sooty
- Its less hot
-
-
- Nitrogen gas
- Oxygen
-
- Components of our can be separated by physical means
- Components are not chemically combined
- Bubble the air into an aqueous solution of calcium hydroxide a white precipitate is formed.
-
-
-
- Alkali metals
- 2:8:5
- R>R2+
It loses electrons when forming ion hence energy level lost resulting to decrease in size. -
- P and W:
P+ + W2- → P2W - T and Y
T4+ + Y-1 → TY4
- P and W:
- S>Q
Q has more valence e- hence stronger metabollic bond Q smaller than S hence stronger metallic bond - M - Show negligible tendencies of gaining or loosing electrons
- S has more valence e- hence better conductor
S forms oxide making it unreactive. -
-
- A- ammonia
B - Calcium oxide - CaO(s) + H2O(l) → Ca(OH)2(aq)
- It is exothermic
- Filtration
- Ammonia
Carbon (iv) oxide - Drying agent in preparation of hydrogen gas
In extraction of Na from NaCl by lowering mo of NaCl - Soften hard water
Used in making glass
- A- ammonia
-
- Iron (III)chloride
- 2Fe(s) + 3Cl2(g) → 2FeCl3(s)
- It sublimes
- To keep mositure at bay since FeCl3 is deliquesent substance
- Aluminium
-
-
Name Formula Solution A Sodium hydroxide NaOH Solution B Zinc Chloride ZnCl2 -
- Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
- 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
- Insert a burning splint in gas jar containing gas C, its extinguished with a pop sound.
-
- Darts on surface
- Melts forming silvery bell
- Hissing sound
- Ignites spontaneously
- Anhydrous salt is salt that doesn't contain water of crystallisation while dry salt is a salt which doesn't have any moisture or water in it.
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 3 Term 2 Opener Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students