INSTRUCTIONS.
Answer all the questions in the spaces provided.
- The grid below shows part of the periodic table. The letters do not represent
the actual symbols. Use it to answer the questions that follow.- How is the atomic radius of element X and Y compared? (2mks)
- Using crosses (x) to represent electrons, draw the atomic structure of element Q. (1mk)
State the period and the group to which element Q belong. (2mks) - The ionic configuration of element G is 2.8 G forms an ion of the type G -1 . Indicate in the grid the position of element G. (1mk)
- To which chemical family does element G belong? (1mk)
- State one use of element U. (1mk)
- Write the equation that would take place when Y is heated with air. (2mks)
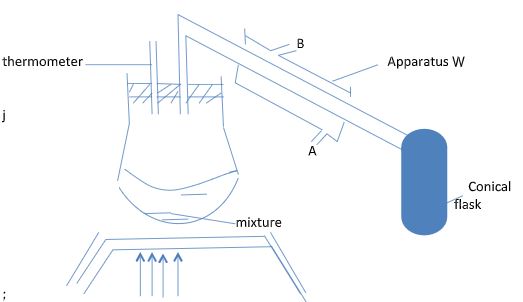
- A student left some crushed fruit mixture which fermented to form water and ethanol with boiling point of 100 O C and 78 O C respectively. The set up of the apparatus below were used to separate the mixt
- Name the apparatus labeled W. (1mk)
- What is the purpose of the thermometer in the set-up? (1mk)
- At what end of the apparatus W would tap water connected? (1mk)
- Which liquid was collected first as a distillable? Explain (2mks)
-
- What is the name given to above method of separating mixture? (1mk)
- State two application of the above method of separating mixtures. (2mks)
- What properties of the mixture make it possible to be separated by the above method? (1mk)
- Name the apparatus labeled W. (1mk)
-
-
- State one use of graphite. (1mk)
- Both graphite and diamond are allotrope of element carbon. Graphite conduct electricity whereas diamond does not. Explain. (2mks)
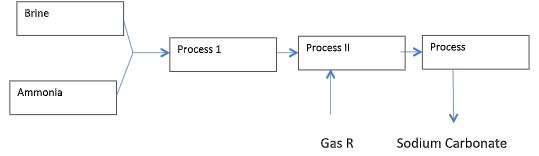
- Below is a simplified scheme of solvery process. Study it and answer the questions that follow.
- Identify gas R. (1mk)
- Write an equation for the process III. (1mk)
- Name the process II. (1mk)
- Give two uses of sodium carbonate. (2mks)
- The diagram below shows a charcoal stove with different region.
- Write an equation for the formation of product B. (1mk)
- How would one prevent the production of product at B? (2mks)
-
- An unknown mass X, of an hydrous potassium carbonate was dissolve in water and the solution made up to 200cm3 . 25cm3 of this solution required 18cm 3 of 0.22M nitric acid for complete neutralization. (K=39,C=12,O=16)
- Write an equation for the reaction that took place (2mks)
- Calculate the number of moles of nitric (V) acid that reacted with anhydrous potassium carbonate. (2mks)
- Calculate the number of moles of anhydrous potassium carbonate that was neutralized by acid. (2mks)
- Determine the value of X. (2mks)
-
- Describe the process by which oxygen can be obtain from air. (4mks)
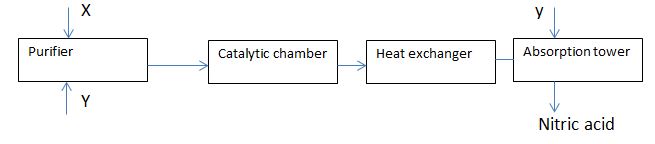
- The flow chart below shows industrial manufacture of nitric (V) acid.
- Identify substance X and Y. (2mks)
- Write an equation for the reaction taking place in the absorption tower. (2mks)
- The flow chart below shows industrial manufacture of nitric (V) acid.
-
- The concentration of acid obtain is 60%. How can this concentration be increased to about 65%. (1mk)
- A factory uses nitric (V) acid and ammonia as the only reaction for the production of a fertilizer. If a mass of 9600kg of fertilizer was produced. Calculate the mass of ammonia gas needed. (N=14, H=1, O=16) (3mks)
- Describe the process by which oxygen can be obtain from air. (4mks)
-
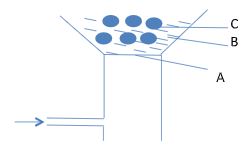
- Sulphur is extracted from underground deposits by process in which three concentric pipes are sink down to the deposit as shown
.
- Name the process represented above. (1mk)
- What is passed down through pipe J? (1mk)
- Name two allotropes of sulphur. (2mks)
- Commercial sulphuric acid has a density of 1.8gcm-3 .
- Determine the molarity of the acid. (3mks)
- Determine the volume of commercial acid in a above that can be used to prepare 500cm3 of 0.2MH2SO4 solution. (3mks)
- Oleum is an intermediate product in the industrial manufacture of sulphuric acid. How is oleum (H2S2O7) converted into sulphuric acid. (1mk)
- Give two use of sulphuric (VI) acid. (2mks)
- Sulphur is extracted from underground deposits by process in which three concentric pipes are sink down to the deposit as shown
- Two reagent that can be used to prepare chlorine gas are manganese(IV) oxide and conc. Hydrochloric acid.
- Write an equation for the reaction. (2mks)
- Give the formula of another reagent that can be reacted with conc. Hydrochloric acid to produce chlorine gas. (1mk)
- Describe how chlorine gas could be dried and collected in the laboratory. (2mks)
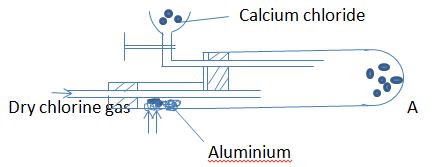
- In an experiment, dry chlorine gas was reacted with aluminium as shown in the diagram below.
- i. Name substance A (1mk)
- Write an equation for the reaction that took place in the combustion tube. (2mks)
- State the function of calcium chloride. (1mk)
-
- Give the properties of substance A. (1mk)
- Name other three substances that behavior as A. (3mks)
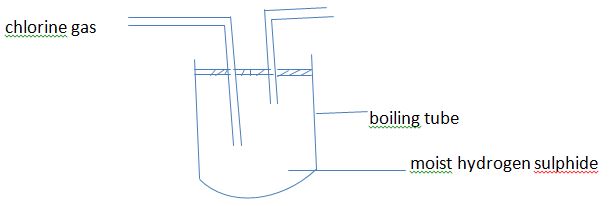
- In an experiment, chlorine was passed into moist hydrogen sulphide in a boiling tube as shown below.
- What observation was made in the boiling tube? (1mk)
- Write an equation for the reaction that took place in the boiling tube. (2mks)
MARKING SCHEME
- The grid below shows part of the periodic table. The letters do not represent the actual symbols. Use it to answer the questions that follow.
- How is the atomic radius of element X and Y compared? (2mks)
- Y has smaller atomic radius than X due to effective nuclear change.
-
- Using crosses (x) to represent electrons, draw the atomic structure of element Q. (1mk)
- E.C of Q 2.8.6
- E.C of Q 2.8.6
- State the period and the group to which element Q belong. (2mks)
- Period (III)
- Group (VI)
- Using crosses (x) to represent electrons, draw the atomic structure of element Q. (1mk)
-
- The ionic configuration of element G is 2.8 G forms an ion of the type G-1. Indicate in the grid the position of element G. (1mk)
- To which chemical family does element G belong? (1mk)
- Halogens
- State one use of element U. (1mk)
- Use in hospital for patient with breathing difficulties.
- Write the equation that would take place when Y is heated with air. (2mks)
Y(s) + O2(g) →YO
Y + N2(g) →Y3N2
- How is the atomic radius of element X and Y compared? (2mks)
- A student left some crushed fruit mixture which fermented to form water and ethanol with boiling point of 100°C and 78OC respectively. The set up of the apparatus below were used to separate the mixture.
- Name the apparatus labeled W. (1mk)
- Lie big condenser
- What is the purpose of the thermometer in the set-up? (1mk)
- Thermometer monitor the temperature of the boiling mixture.
- At what end of the apparatus W would tap water connected? (1mk)
- At A where cold water get in.
- Which liquid was collected first as a distillable? Explain (2mks)
- Ethanol it has lower boiling point compared to water.
- What is the purpose of the thermometer in the set-up? (1mk)
- Lie big condenser
-
- What is the name given to above method of separating mixture? (1mk)
- fractional distillation
- State two application of the above method of separating mixtures. (2mks)
- Fractional distillation of crude oil in an oil refinery.
- Fractional distillation of air.
- What properties of the mixture make it possible to be separated by the above method? (1mk)
- Have different boiling point
- What is the name given to above method of separating mixture? (1mk)
- Name the apparatus labeled W. (1mk)
-
-
- State one use of graphite. (1mk)
- Use as a lubricant, making of pencil.
- Both graphite and diamond are allotrope of element carbon. Graphite conduct electricity whereas diamond does not. Explain. (2mks)
- Graphite uses three of its 4 valence electrons in bonding hence delocalize electron is responsible for conducting electricity while diamond uses all 4 valence electron in bonding.
- State one use of graphite. (1mk)
- Below is a simplified scheme of solvery process. Study it and answer the questions that follow.
- Identify gas R. (1mk)
CO2 - Write an equation for the process III. (1mk)
2NaHCO3(s) —heat→ Na2CO3(s) + H2O(i) + CO2(g) - Name the process II. (1mk)
- Filtration
- Identify gas R. (1mk)
- Give two uses of sodium carbonate. (2mks)
- Manufacture of glass
- Manufacture of paper, water softening
- The diagram below shows a charcoal stove with different region.
- Write an equation for the formation of product B. (1mk)
CO2(g) + C(g)→CO(g) - How would one prevent the production of product at B? (2mks)
- Burn charcoal in sufficient oxygen
- Carbon oxidized to Co2.
- Write an equation for the formation of product B. (1mk)
-
- An unknown mass X, of an hydrous potassium carbonate was dissolve in water and the solution made up to 200cm3. 25cm3 of this solution required 18cm3 of 0.22M nitric acid for complete neutralization. (K=39,C=12,O=16)
- Write an equation for the reaction that took place (2mks)
K2CO3(s) + 2HNO3(aq) →2KNO3 + CO2(g) + H2O(l) - Calculate the number of moles of nitric (V) acid that reacted with anhydrous potassium carbonate. (2mks)
0.22 mole of HNC3 = 1000cm3
18cm3
18cm x 0.22 mole/100
= 0.00396 moles - Calculate the number of moles of anhydrous potassium carbonate that was neutralized by acid. (2mks)
Mole ratio 1:2
0.00396/2
= 0.00198 moles - Determine the value of X. (2mks)
25cm3 = 0.00198 mole
100cm3
= 1000/25x 0.00198
= 0.0792M
Mass x = RFM x Molarity
138 x 0.792
10.9g
- Write an equation for the reaction that took place (2mks)
-
- Describe the process by which oxygen can be obtain from air. (4mks)
- Pass air through purifier to remove dust particles be electrostatic process.
- Pass the gas via conc. NaOH to remove CO2.
- Pass the remaining gas via condenser at 25°C to remove water vapour.
- Cool it to liquefy it then fractional distillation to obtain oxygen at -183°C.
- The flow chart below shows industrial manufacture of nitric (V) acid.
- Identify substance X and Y. (2mks)
X – Ammonia
Y – Air - Write an equation for the reaction taking place in the absorption tower. (2mks)
NO2 + H2O + O2 →HNO3
accept NO2 + H2O →HNO + HNO2
- Identify substance X and Y. (2mks)
-
- The concentration of acid obtain is 60%. How can this concentration be increased to about 65%. (1mk)
- Through fractional distillation
- A factory uses nitric (V) acid and ammonia as the only reaction for the production of a fertilizer. If a mass of 9600kg of fertilizer was produced. Calculate the mass of ammonia gas needed. (N=14, H=1, O=16) (3mks)
HNO3(aq) + NH3(aq) → NH4NO3(aq)
RFM of NH4NO3 = 80g
If 80g NH4NO3 → 17g NH3
960000g
960000 x 17/80 x 1000
2040kg
- The concentration of acid obtain is 60%. How can this concentration be increased to about 65%. (1mk)
- Describe the process by which oxygen can be obtain from air. (4mks)
-
- Sulphur is extracted from underground deposits by process in which three concentric pipes are sink down to the deposit as shown.
- Name the process represented above. (1mk)
- Frasch process
- What is passed down through pipe J? (1mk)
- Hot compressed air
- Name two allotropes of sulphur. (2mks)
- Monoclinic
- rhombic
- Name the process represented above. (1mk)
- Commercial sulphuric acid has a density of 1.8gcm-3.
- Determine the molarity of the acid. (3mks)
RFM H2SO4 =98
Number of moles 1.8/98 = 0.018737 mole
1cm3=0.01837m
1000cm
1000 x 0.01837/1
= 18.3M - Determine the volume of commercial acid in a above that can be used to prepare 500cm3 of 0.2MH2SO4 solution. (3mks)
M1V1 = M2V2
18.3V1= 0.2 x 500
V1 = 5.46cm3 - Oleum is an intermediate product in the industrial manufacture of sulphuric acid. How is oleum (H2S2O7) converted into sulphuric acid. (1mk)
- By dissolving in water
- Give two use of sulphuric (VI) acid. (2mks)
- An electrolyte in car batteries
- Manufacture of paints
- Determine the molarity of the acid. (3mks)
- Sulphur is extracted from underground deposits by process in which three concentric pipes are sink down to the deposit as shown.
- Two reagent that can be used to prepare chlorine gas are manganese(IV) oxide and conc. Hydrochloric acid.
-
- Write an equation for the reaction. (2mks)
Mno2 + 4HCl →MnCl2 + 2H20 + Cl2
Must be balance
State symbols - Give the formula of another reagent that can be reacted with conc. Hydrochloric acid to produce chlorine gas. (1mk)
KMnO4 - Describe how chlorine gas could be dried and collected in the laboratory. (2mks)
- Can be dried by passing through a was bottle of conc. H2SO4 then collected be downward delivery.
- Write an equation for the reaction. (2mks)
- In an experiment, dry chlorine gas was reacted with aluminium as shown in the diagram below.
- Name substance A (1mk)
- Aluminium chloride
- Write an equation for the reaction that took place in the combustion tube. (2mks)
2Al(s) + 3Cl2 (g) →2AlCl3(g) - State the function of calcium chloride. (1mk)
- Prevent moisture from entering the flask.
- Name substance A (1mk)
-
- Give the properties of substance A. (1mk)
- It sublimes
- Name other three substances that behavior as A. (3mks)
- Solid Co2
- Benzoic acid
- Iodine
- Give the properties of substance A. (1mk)
-
- In an experiment, chlorine was passed into moist hydrogen sulphide in a boiling tube as shown below.
- What observation was made in the boiling tube? (1mk)
- Yellow solid deposit of sulphur on the wall of the boiling tube.
- Write an equation for the reaction that took place in the boiling tube. (2mks)
H2S(g) + Cl2(g) →2HCl(g) + S (s)
Must be balance and state symbolys
- What observation was made in the boiling tube? (1mk)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY PAPER 2 Questions and Answers - Form 3 End Term 1 Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students