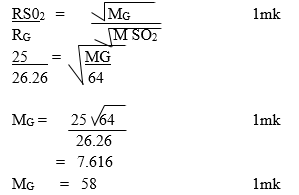ANSWER ALL THE QUESTIONS.
- State two reasons why we use the non-luminous flame for leading in a laboratory instead of using the luminous flame. (1mk)
- Chlorine has two isotopes with atomic mass 35 and x occurring in the ratio 3:1 respectively. The relative atomic (R.M.A) of chlorinw is 35.5. Determine the value of x. (3mks)
- The use of cfc5 has been linked to the depletion of the ozone layer.
a) What does CFC stand for? (1mk)
b) Explain the problem associated with the depletion or the ozone layer. (1mk)
c) State another environmental problem caused by CFC5. (1mk) - In an experiment to prepare Nitrogen (1) oxide, ammonium nitrate was gently heated in a flask.
a) Write the equation for the reaction that took place in the flask. (1mk)
b) State and explain how the gas was collected. (1mk)
c) A sample of the gas was tested with damp blue and red litmus paper what observations were made. (1mk) - During an experiment sulphur (IV) oxide gas was formed to diffuse through a certain pore at a rate of 25cm3 per minute. When the experiment was repeated under the same conditions with another gas G, gas G was found to diffuse through the same pore at a rate of 26.26cm3 per minute. Work out the molecular mass of Gas G. (0=16, 5=32) (3mks)
- Element Y whose atomic number11 react with chlorine gas to form a compound.
a) Name the group and period to which Y belongs. (1mk)
b) Write an equation for the reaction. (1mk) - Draw all structural formulas for all the isomers with molecular formula C2H3CL3. (2mks)
- Calculate the volume of 0.6M sulphuric (VI) acid solution needed to neutralize 30cm3 of 0.2M potassium hydroxide. (2mks)
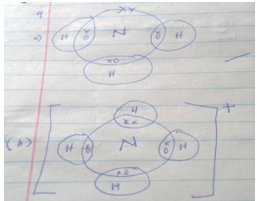
- Use dot (.) and crosses (x) to show the bonding of the following compounds.
a) NH3 (1mk)
b) NH4+ (1mk) - Analysis of a compound showed that it had the following composition: 69.42% carbon, 4.13% hydrogen and the rest oxygen. If the molecular formula of the compound (C=12, O=16, H=1). (3mks)
- A reference book states that the solubility of CuSO4 in water at 15oc is 19g/100g of water. What is meant is meant by this statement. (1mk)
- State two uses of hydrogen gas. (2mks)
- Explain how a solid mixture of sulphure and potassium Chloride can be separated into solid sulphur and potassium chloride. (3mks)
- Aqueous ammonia was added to a solution copper (ii) sulphate dropwise until in excess. State the observations made when
a) A few drop of aqueous ammonia were added.(1mk)
b) Excess aqueous ammonia was added. (1mk) - By use of chemical equations distinguish the reaction of magnesium with water and magnesium with steam. (2mks)
- The table below gives the number of electrons, protons and neutrons in substances X, Y, and Z.
a)Which letter represents an ion? (1mk)Substance
Electrons
Protons
Neutrons
X
10
10
10
Y
10
8
10
Z
8
8
8
b) Which of the substances are isotopes? Give a reason. (2mks) - a) What is meant by the terms.
- Element (1mk)
- Atomic number (1mk)
- The chart below shows a scheme or reactions involving a sample of solution N. Study it and answer the questions that follow.
a) Identify the cation and the anion in solution N, (2mks)
b) Write an ionic equation to show how Q is formed. (1mk) - Name the process where
Solid carbon (IV) oxide (dry ice) changes directly into gas. (1mk) - When carbon (IV) oxide gas was passed through aqueous calcium hydrogen a white precipate was formed.
a) Write an equation for the reaction that took place. (1mk)
b) State and explain the changes that would occur when excess carbon (iv) oxide gas is bubbled through the white precipitate. (2mks) - Give the names of the following compounds
- Explain why burning magnesium continue to burn in a gas jar full of sulphure (iv) oxide while humming splint would be extinguished. (3mks)
- When hydrogen sulphide gas was bubbled into aqueous solution of iron (iii) chloride a yellow precipitate was formed.
a) State another observation that was made. (1mk)
b) Write an equation for the reaction that took place. (1mk)
c) What type of reaction was undergone by hydrogen sulphade gas in this reaction? (1mk) - a) What is allotropy (1mk)
b) Name two allotropes of carbon. (2mk) - Ammonium sulphate is a fertilizer produced by passing ammonia gas into concentrated sulphure (VI) acid. Calculate the mass in kg of sulphure (VI) acid required to produce 25kg or the fertilizer. (s=32, 0=16, N=14,H=1) (3mks)
- The reaction between hot concentrated Sodium hydrogen and chlorine gas produces sodium chloride (v), sodium chloride and water.
- Write the equation for the reaction. (1mk)
- Give one use of sodium chlorate (v). (1mk)
- Explain why a solution of hydrogen chloride gas in methylbenzene does not conduct electricity but solution of a gas in water conduct electricity. (2mks
- Below is a sketch of a reaction profile. Study it and then answer the question that follows.
State and explain the type of reaction represented by the profile. (2mks) -
- What are amphoteric oxides? (1mk)
- Give a chemical formula example of an amphoric oxide. (1mk)
- Calcium oxide can be used to dry ammonium gas.
- Explain why Calcium oxide is not used to dry hydrogen Chloride gas. (2mks)
- Name one drying agent of hydrogen chloride. (1mk)
- When an organic compound Y is reacted aqueous Sodium carbonate it produces carbon (iv) Oxide. Y reacts within propanal to form a pleasant smelling compound whose formula is.
O
|
CH3CH2 — C — O — CH2CH2CH3- Name and draw the structure formula of compound Y. (2mks)
- What is the name given to the group of compound to which Z belongs? (1mk)
- Element X and Y have atomic numbers 20 and 8 respectively.
- Write the electron arrangement of their ions. (2mks)
- Write the formula of the compound formed between X and Y. (1mk)
MARKING SCHEME.
- — It is very hot.
— Does not from soot each ½ mks - R.A.M=35.5
35.5= ¾ ×35 + ¼ × x (1mk)
142=105 + x (1mk)
X = 37 (1mk) - (a) Chlorofluorocarbon (1mk)
(b) When ozone layer is depleted high energy radiation reach the earth and may cause cancer to human beings. (1mk)
(c) Global warming. (1mk)
heat - (a) NH4NO3(S) → N2O(3)+2H2O(3) (1mk)
(b) Over warm water, it’s fairly soluble in cold water (1mk)
(c) Both red blue litmus paper were not affected / did not change. (1mk)
- (a) Group I (½ mk)
Period 6 (½ mk)
(b) 2Y(S) + Cl2 (g) → 2YCl(s) (1mk - C2H3Cl3
(Each 1mk)
- H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) +2H2O(l)
Moles of KOH 30×0.2
1000
= 6/1000
=0.006 Mmoles (1mk)
Moles of H2SO4 reacting ratio 1:2
= moles of H2SO4 =0.006
2
= 0.003 moles (1mk)
0.003 = 0.6×V
0.6
= 3/0.6 = 5cm3 (1mk) -
- C H O
% Composition 42 4.13 26.45 ½ mk
R.A.M 12 1 16
MOLES 5.785 4.13 1.653 ½ MK
MOLES RATIO 5.785 4.13 1.653 = 1
1.653 1.653 1.653
3.5 2.5
Whole number ratio 3.5×2=7 2.5×2=5 1×2=2 ½ mk
EF C7H5O2 ½ mk
(C7H5O2)n =242
(84+5+32)n=242
121n=242
n =2 ½ mk)
MF= (C7H5O2)2
= C14H10O4 ½ mk - It means that a maximum of 19g of cuso4 dissolves in 100g of water at 150 1mk
-
- Manufacture of O2Hcl(aq)
- Manufacture of ammonia
- Hardening of oils into fats.
- In hydrogen flame which is used in welding (any two 1mk)
- Add water to the mixture potassium chloride dissolves it is ionic while Sulphur is molecular. Filter the mixture to obtain Sulphur as residue and potassium chloride as filtrate. Evaporate the filtrate to obtain solid KCL.
-
- Pale blue precipitate is formed. 1mk
- Deep blue solution will be formed. 1mk
- with water
Mg(s) +2H2O (l) → Mg (OH)2 (aq) + H2 (g) 1mk
With steam
Mg(s) + H2O(g) → MgO (S) + H2 (g) 1mk -
- Y 1mk
- Y and Z (1mk) because they have the same (1mk) number of protons but different number of neutrons.
-
-
- An element is a substance made of one kind of atom and cannot be split into simpler substance by chemical means. 1mk
- Atomic number is the number of protons in an atom of an element.
- Ti2 (SO4)3
-
- Anion SO42- 1mk
Cation Zn2+ 1mk
Ba2+(aq) + SO2+4 (aq) → BaSO4(s) 1mk
- Sublimation 1mk
- Ca(OH)2(s) + CO2(g) → CaCO3(s) +H2O
The white precipitate would dissolve due to formation of soluble calcium hydrogen carbonate. 1mk - 2, 2 –dimethylpropane.
Pent-2-yne - Burning magnesium produces a lot of heat which breaks the bond between sulphur and oxygen in SO2. Magnesium then uses the oxygen which was broken from sulphure to continue burning; a burning splint does not produce a lot of heat.
- Yellow solid (sulphur) is deposited. 1mk
H2S (s) +2FeCl3 (aq) → 2Fecl2 (aq) + S (s) +2Hcl (aq)
H2S is oxidized to sulphur 1mk - This existence of an element in more than one form in the same physical state. 1mk
Diamond 1mk
Graphite 1mk
- 2NH3 (s)+ H2SO4(l) → (NH4)2SO4 (aq)
R.M.M of (NH4)2 SO4 = 28+8+32+64
=132
Moles required to produce 25kg = 25000g
Moles =25000g =189.39 moles 14mks
132
Moles of H2SO4 required =189.39 moles
R.M.M of H2SO4 =98
Mass = 98× 189.39 1mk
=18560g
=18.56kg 1mk - 6NaOH(aq) + 3Cl2 (g) → NaClO3 + 5Nacl(aq) + 3H2O(l)
Manufacture of bleaching agents. - Methylbenzene is a non- polar compound hence hydrogen chloride in it does not ionize but exist as a molecule substance but in water hydrogen chloride ionizes to give H+ and cl- ions that’s why it conduct electricity in water.
- It is endothermic 1mk . This is because the products are heavy more than energy than the reactants. 1mk.
- These are oxides which react with both acids and alkalis. 1mk
Al2O3, ZnO and Pbo. Any two - It would react with HCl(g) since it is basic and Hcl is acidic to form calcium chloride and water.
Concentrated H2SO4
Anhydrous calcium chloride. -
- Propanoic acid 1mk
H H O 1mk
| | ||
H —C — C — C — OH
| |
H H - Esters
- Propanoic acid 1mk
-
- X2+8.8
Y2- 2.8 - XY
- X2+8.8
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY PAPER 1 - FORM 4 END TERM 1 EXAMS 2020.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students