INSTRUCTIONS TO CANDIDATES
- Answer all the questions in the spaces provided in the question paper.
- You are NOT allowed to start working with the apparatus for the first 15 minutes of the 2 ¼ hrs allowed for this paper. This time is to enable you to read the question paper and make sure you have all the chemicals and apparatus that you may need.
- All working must be clearly shown where necessary.
- You are provided with
- Anhydrous sodium carbonate solid x.
- Distilled water.
- 2m Hydrochloric acid solution
PROCEDURE I
- Place 50.0ml of water in 250ml plastic beaker.
- Note the temperature of the water and record it in the table I below.
- Add all the solid X provided to the water in the beaker, stir gently with the thermometer and record the final temperature of the solution in the table I below. Keep the resulting solution for procedure 2.
TABLE I
(2 mks)Final temperature (0C)
Initial temperature (0C)
Change in temperature (0C)
- What is the enthalpy change for the reaction? (Assume the density of solution is 1g/cm3, and specific heat capacity is 4.2 Jg-1 K-1). (2 mks)
PROCEDURE II
Transfer the contents of the beaker into 250ml volumetric flask. Rinse both the beaker and the thermometer with distilled water and ass this water into the solution in the volumetric flask. Add more water to make up to the mark. Label this solution as solution X. fill the burette with solution A. Using a pipette place 25.0ml of solution X into a conical flask. Add 3 drops of methyl orange indicator and titrate with solution A. record your readings in table II below. Repeat the titration two more times and complete the table.
TABLE II
(3 mks)Experiment
Final burette reading (cm3)
Initial burette reading (cm3)
Volume of solution A used (cm3)
- Calculate average volume of solution A used. (1 mk)
- the number of moles of solution A used. (1 mk)
- The number of moles of solution X that reacted with the number of moles of solution A in (c) above. (1 mk)
- The number of moles of solid X used in procedure I. (1 mk)
- Molar heat of solution of anhydrous sodium carbonate. (2 mks)
- You are provided with:
- A solution of sodium hydroxide labeled B.
- A solution of sulphuric(vi)acid labeled C.
You are required to determine the concentration of the alkali using the following procedure.
PROCEDURE:
- Place 40cm3 of sodium hydroxide solution into a 250 ml plastic beaker.
- Measure 60cm3 of sulphuric (vi) acid solution.
- Determine the temperature of sodium hydroxide solution at half a minute intervals for two minutes and record it in the table below.
- At 2 ½ minutes, place the 60cm3 of solution C into the plastic beaker while stirring and resume taking the temperature in the 3rd minute.
- Complete the table below.
Time in minutes
0
½
1
1½
2
2½
3
3½
4
Temperature in 0C
Χ
(3 mks)Time in minutes
4½
5
5½
6
6½
7
Temperature in 0C
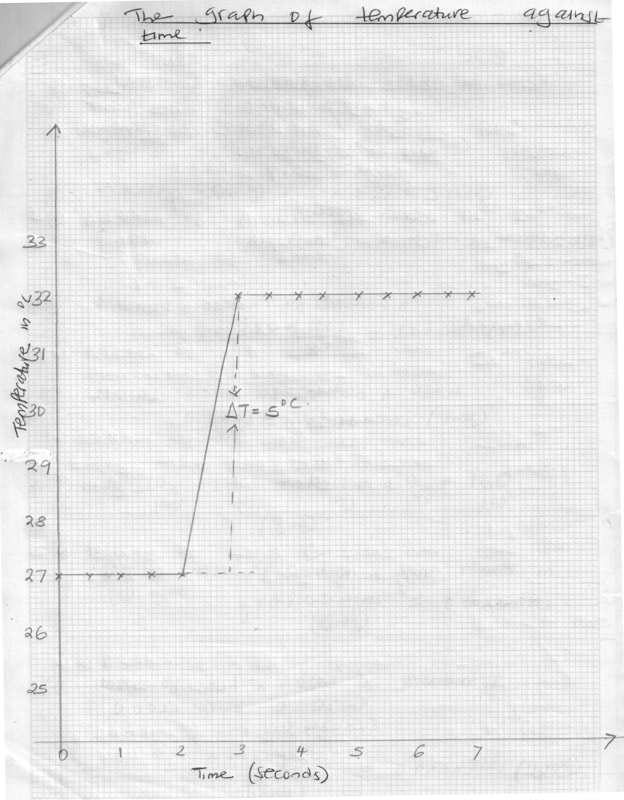
- Plot a graph of temperature against time. (3 mks)
- From the graph, determine the highest temperature change. (1 mk)
- Determine the heat evolved in this experiment (Density of solution = 1 g/cm3 specific heat capacity of solution = 4.2 Jg-1 K-1) (2 mks)
- Given that the molar heat of neutralization is 56KJ/mole, determine the number of moles of sodium hydroxide used in the neutralization reaction above. (2 mks)
- Determine the molarity of sodium hydroxide. (2 mks)
- You are provided with solid K. carry out the following tests and write your observations and inferences in the spaces provided.
TEST
OBSERVATION
INFERENCE
(a)
Place a spatula full of sample K in a clean dry test tube. Heat gently and then strongly.
(1 mk)
(1 mk)
(b)
Put the remaining solid K in a boiling tube. Add about 8cm3 of distilled water. Shake well and divide the solution into 3 portions.
(i)
To the first portion add 3 drops of sodium hydroxide solution and then excess.
(1 mk)
(1 mk)
(ii)
To the second portion add 3 drops of ammonia solution and then excess.
(1 mk)
(1 mk)
(iii)
To the third portion add 3 drops of Barium nitrate followed by 3 drops of nitric acid.
(1 mk)
(1 mk)
(c)
You are provided with solid P. carry out the tests below and record your observations and inferences.
(i)
Place half spatula of solid P in a non-luminous flame of a Bunsen burner.
(1 mk)
(1 mk)
(ii)
Dissolve the remaining solid in water and divide into two portions
(I)
Add 3 drops of universal indicator to the 1st portion and determine the PH of the solution.
(1 mk)
(1 mk)
(II)
To the 2nd portion add a little sodium hydrogen carbonate
(1 mk)
(1 mk)
MARKING SCHEME
TABLE 1
- Initial temperature – ½ mk.
Final temperature – ½ mk.
Change in temperature – 20C. (1 mk)
(a) Enthalpy change = -50 x 4.2 x 2J. (1 mk)
= -420J (1 mk)
(1/2 mk penalty for missing negative sign)
(b) Average volume of solution A.
22.9+23.0+23.1 = 23.0 cm3(1 mk)
3
(c) No of moles of solution A used.
0.2×23 = 0.0046 moles(1 mk)
1000
(d) Na2CO3(aq) + 2HCl(aq) → 2Nacl(aq) + CO2(g) + H2O(l)
1:2 (1/2 mk)
No of moles of solution X that reacted in (c) above.
½ X 0.0046 moles = 0.0023 moles. (1/2 mk)
(e) Moles of solid X used in procedure I
0.0023 moles → 25cm3
→ 250 cm3
( ½ mk)
(f) molar heat of solution of Na2CO3
0.023 moles -420J (½ mk)
1 mole -420 J (½ mk)
0.023
= -18.260.86-J
= 18.2608 KJmol-1 (1 mk) -
Time in minutes
0
½
1
1 ½
2
2 ½
3
3 ½
Temperature in 0C
27.0
27.0
27.0
27.0
27.0
χ 32.0
32.0
Time in minutes
4
4 ½
5
5 ½
6
6 ½
7
Temperature in 0C
32.0
32.0
32.0
32.0
32.0
32.0
32.0
CT = 1mk
Trend = ½ mk
Use of decimals = 1 mk
1st reading = I 20C S.V ½ mk
(a) Graph
Labeled Axis – (1/2 mk for each)
Plotting – 1 mk
Shape – 1 mk
(b) DT = 50C. (1 mk)
(c) Heat change =
Total volume of solution = 40 + 60 = 100cm3 (½ mk)
Mass of solution = 100g
Heat change = -100 X 4.2 X 5J (½ mk)
= -2100 J (1 mk)
= -2.1 KJ
(d) Heat of neutralization – 56KJ/mole.
1 mole produces 56 KJ
? 2.1 KJ
1×2.1 =0.0375 moles
56
(1 mk) (1 mk)
(e) molarity of NaoH
V – 40cm3
Moles – 0.0375
No of moles =
0.0375 =
M = (1 mk)
= 0.9375M. (1 mk) -
TEST
OBSERVATIONS
INFERENCE
(a) Heating solid k.
A colourless gas that turns moist red litmus paper to blue is produced. (1 mk)
NH4+ present (1 mk)
(b) (i) Addition of NaOH
A white ppt (½ mk) which dissolves in excess. (½ )
Al3+, Zn2+ or Pb2+ present
All 3 – 1mk
2 only – ½ mk
½ mk penalty for a wrong ion
(ii) Addition of Ammonia solution
A white ppt ½ mk which dissolves in excess ½ mk
Al3+, Zn2+ or Pb2+ present.
All 3 – 1mk
2 only – ½ mk
½ mk penalty for a wrong ion
(iii) Addition of Ba(NO3)2 then HNO3
A white ppt ½ mk which dissolves in excess ½ mk
Zn2+ present (1 mk)
(c) (i) Burning of solid P
Solid P burns with a sooty flame (1 mk)
C = C or
-C- C – present
½ mk for one
(ii) (a) Addition of universal indicator
PH of 4 (1 mk)
Solution is weakly acidic (1 mk)
(b) Addition of NaHCO3
Effervescence present
H+ present
Download CHEMISTRY PAPER 3 - FORM 4 END TERM 1 EXAMS 2020.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

