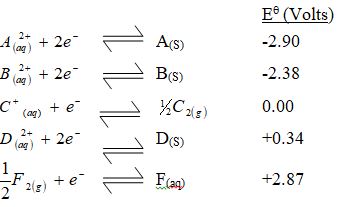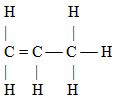CHEMISTRY
FORM 4
END TERM EXAMS
TERM 1 2021
PAPER 2
(THEORY)
TIME 2 hrs
INSTRUCTIONS
- Answer ALL the questions in the spaces provided.
- All working must be clearly shown where necessary.
- Mathematical tables and silent electronic calculators may be used.
-
- Study the table below and answer the questions that follow.
Element Atomic
radius (nm)Ionic
radius (nm)Formula
of oxideMelting point
of oxide (ºC)A 0.364 0.421 A2O -119 B 0.830 0.711 BO2 837 E 0.592 0.485 E2O3 1466 G 0.381 0.446 G2O5 242 J 0.762 0.676 JO 1054 - Which elements are non-metals? Give a reason. (3 marks)
- Explain why the melting point of the oxide of E is higher than that of the oxide of G. (2 marks)
- Give two elements that would react vigorously with each other. Explain your answer. (2 marks)
- Study the information below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
Element Electronic
ConfigurationIonization energy KJ/mol 1st I.E 2nd I.E. X 2.2 900 1800 Y 2.8.2 736 1450 Z 2.8.8.2 590 1150 - What chemical family does the elements X, Y and Z belongs? (1 mark)
- What is ionization energy? (1 mark)
- The 2nd ionization energy is higher than the 1st ionization energy of each element. Explain. (1 mark)
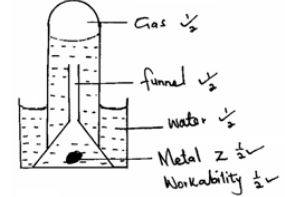
- When a piece of element Z is placed in cold water, it sinks to the bottom and effervescence of a colourless gas that burns explosively is produced. Use a simple diagram to illustrate how this gas can be collected during this experiment. (2 marks)
- Study the table below and answer the questions that follow.
-
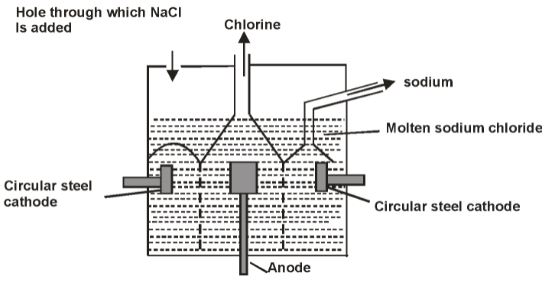
- Below is a simplified diagram of the Down’s cell used for the manufacture of sodium. Study it and answer the questions that follow.
- What material is the anode made of? Give a reason (2mks)
- What precautions are taken to prevent chlorine and sodium from re-combining? (1mk)
- Write an ionic equation for the reaction in which chlorine gas is formed. (1mk)
- In the Downs process above a certain salt is added to lower the melting point of sodium chloride from about 800ºc to about 600ºc.
- Name the salt that is added. (1mk)
- State why it is necessary to lower the temperature. (1mk)
- Explain why aqueous sodium chloride is not suitable as an electrolyte for the manufacture of sodium in the Downs process. (2mks)
- Sodium metal reacts with air to form two oxides. Give the formulae of the oxides. (1mk)
- Below is a simplified diagram of the Down’s cell used for the manufacture of sodium. Study it and answer the questions that follow.
-
- One mole of heptane was thermally cracked, two hydrocarbons Q and P were formed.
Q was an alkene molecule with three carbon atoms.- Give the molecular formula of.
- Q (1 mark)
- P (1 mark)
- Write the structural formula of Q. (1 mark)
- Name the compound formed when Q undergoes self addition reaction. (1 mark)
- State one disadvantage of using the product named in a(iii) above. (1 mark)
- Cracking can also be achieved using less amount of heat in the presence of a catalyst. Name one catalyst that is often used. (1 mark)
- Give the molecular formula of.
- An organic compound J has the following percentage by mass, carbon 64.86%, hydrogen 13.51% and the rest is oxygen. The relative molecular mass of the compound is 74. C = 12, O = 16, H = 1)
- Work out the molecular formula of compound J. (3 marks)
- To which homologous series does compound J belong? (1 mark)
- Write a balanced chemical equation for the reaction that occurs when compound J reacts with sodium metal. (1 mark)
- Name the type of reaction indicated in b(iii) above. (1 mark)
- Name the organic compound formed when J reacts with excess acidified potassium managanate (VII). (1 mark)
- One mole of heptane was thermally cracked, two hydrocarbons Q and P were formed.
- 2.5g of a pure metal carbonate MCO3 was reacted with excess 2M nitric (V) acid. The volume of carbon (IV) oxide evolved was measured and recorded at 10 second intervals. The results were recorded as shown in the table below.
Volume of gas (cm³) 0 90 150 210 280 340 390 450 480 480 480 Time (seconds) 0 10 20 30 40 50 60 70 80 90 100 -
- On the grid provided, plot a graph of volume (vertical axis) against time – Label it curve A. (3 marks)
- From your graph determine the rate of reaction between 25 seconds and 40 seconds. (2 marks)
- On the same axes, sketch a curve that would be obtained if the same experiment was repeated using excess 1M nitric (V) acid. Label it curve B. (1 mark)
- Give that carbon (IV) oxide was measured at room temperature and pressure, work out the relative atomic mass of metal M. (Molar gas volume at r.t.p = 24dm³, C = 12, O = 16). (3 marks)
- When bromine is dissolved in water the equilibrium shown below is established.
State and explain the observation that would be made if aqueous sodium hydroxide was added to the equilibrium mixture. (2 marks)
-
-
- Use the standard electrode potentials for elements A, B, C, D and F given below to answer the questions that follow.
- Which element is likely to be hydrogen? Give a reason for your answer. (2 marks)
- What is Eθ of the strongest reducing agent? (1 mark)
- Calculate the e.m.f of the cell that would be formed when half cells of B and D are combined. (1 mark)
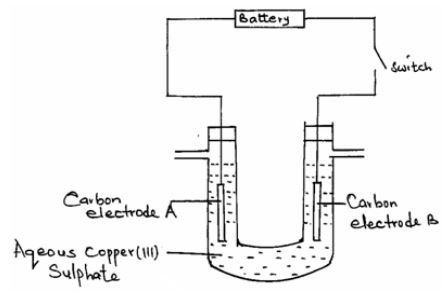
- Aqueous copper (II) sulphate was electrolysed using the set up below.
- When the switch was closed a gas was produced only at electrode B. Which electrode is the anode? (1 mark)
- Write the half equation for the reaction occurring at electrode B. (1 mark)
- What happens to the PH of the electrolyte during electrolysis? Explain. (2marks)
- If carbon electrodes were replaced with copper electrodes in the cell above, write the equation of the reaction that would occur at the anode. (1 mark)
- During electrolysis of aqueous copper (II) sulphate using copper electrodes a current of 0.2 amperes was passed through the cell for 5 hours. Determine the change in mass of the cathode that occurred as a result of the electrolysis process. (Cu = 64, IF = 96500 coulombs). (3 marks)
- Use the standard electrode potentials for elements A, B, C, D and F given below to answer the questions that follow.
-
- State Hess’s law. (1 mark)
- Use the following information to answer the questions that follow:
C(S) + O2(g) → CO2(g) ΔH = -393KJ/mol
H2(g) + ½O2(g) → H2O(g) ΔH = -296KJ/mol
C4 H10 + 13/2O2(g) →4CO2(g)+ 5H2O ΔH = -2877KJ/mol- Draw an energy cycle diagram relating heat of formation and combustion of butane. (2 marks)
- Calculate the heat of formation of butane. (3 marks)
- Distinguish between hydration energy and lattice energy. (2 marks)
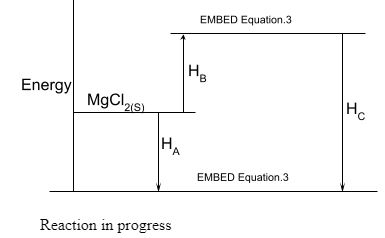
- The diagram below shows an energy level diagram for the formation of magnesium chloride. Study it and answer the questions that follow.
- State the enthalpy changes represented by
- (½ mark)
- (½ mark)
- (½ mark)
- What is the relationship between ΔHA, ΔHBA and ΔHC. (½ mark)
- State the enthalpy changes represented by
-
- Define heat value of a fuel. (1 mark)
- Give two reasons why wood and charcoal are chosen for domestic heating. (2 marks)
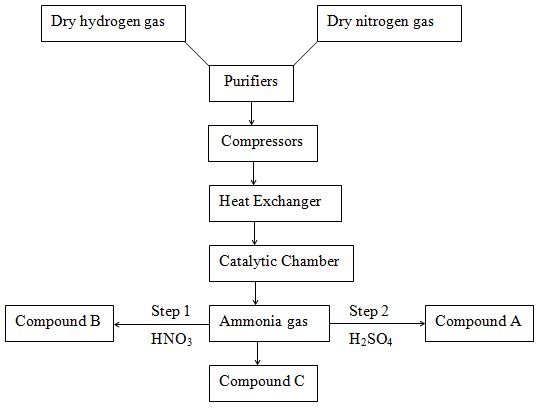
- The flow chart below shows the large scale manufacture of ammonia gas and some ammonium compounds. Study it and answer the questions that follow.
- What are the sources of the following raw materials?
- Hydrogen gas. (1 mark)
- Nitrogen gas. (1 mark)
- What optimum conditions are needed during the manufacture of ammonia in the
- Compressor. (1 mark)
- Catalytic chamber. (1 mark)
- Why should the gas be passed through the compressor. (1 mark)
- Write an equation for the reaction that occurs in Step 1. (1 mark)
- Write the formula of the compound B. (1 mark)
- Calculate the percentage of nitrogen in compound A. (2 marks)
- What observation would be made if compound C was added to a sample suspected to contain copper (II) ions dropwise then in excess? (2 marks)
- What are the sources of the following raw materials?

MARKING SCHEME
- Study the table below and answer the questions that follow.
Element Atomic
radius (nm)Ionic
radius (nm)Formula
of oxideMelting point
of oxide (ºC)A 0.364 0.421 A2O -119 B 0.830 0.711 BO2 837 E 0.592 0.485 E2O3 1466 G 0.381 0.446 G2O5 242 J 0.762 0.676 JO 1054 - Which elements are non-metals? Give a reason. (3 marks)
- A and G
- The ionic radius is greater than the atomic radius
- Explain why the melting point of the oxide of E is higher than that of the oxide of G. (2 marks)
- The oxide of E contains strong electrostatic forces since it is ionic while the oxide of G has weak intermolecular forces // van der waals forces since it is molecular.
- The oxide of E contains strong electrostatic forces since it is ionic while the oxide of G has weak intermolecular forces // van der waals forces since it is molecular.
- Give two elements that would react vigorously with each other. Explain your answer. (2 marks)
- B and A.
- B is the most reactive metal while A is the most reactive non metal
// B has the largest atomic radius so loses electrons most readily while A has the smallest atomic radius so it gains electrons most readily.
- Which elements are non-metals? Give a reason. (3 marks)
- Study the information below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
Element Electronic
ConfigurationIonization energy KJ/mol 1st I.E 2nd I.E. X 2.2 900 1800 Y 2.8.2 736 1450 Z 2.8.8.2 590 1150 - What chemical family does the elements X, Y and Z belongs? (1 mark)
- Alkaline – earth metals
- Alkaline – earth metals
- What is ionization energy? (1 mark)
- The amount of energy required to remove an electron from an atom when in gaseous state.
- The amount of energy required to remove an electron from an atom when in gaseous state.
- The 2nd ionization energy is higher than the 1st ionization energy of each element. Explain. (1 mark)
- 2nd ionization energy involves removal of an electron from a positively charged ion while 1st ionization energy involves removal of an electron from a neutral atom.
- 2nd ionization energy involves removal of an electron from a positively charged ion while 1st ionization energy involves removal of an electron from a neutral atom.
- When a piece of element Z is placed in cold water, it sinks to the bottom and effervescence of a colourless gas that burns explosively is produced. Use a simple diagram to illustrate how this gas can be collected during this experiment. (2 marks)
- What chemical family does the elements X, Y and Z belongs? (1 mark)
- Study the table below and answer the questions that follow.
-
- Below is a simplified diagram of the Down’s cell used for the manufacture of sodium. Study it and answer the questions that follow.
- What material is the anode made of? Give a reason (2mks)
- Carbon / graphite
- It does not react with chlorine formed
- What precautions are taken to prevent chlorine and sodium from re-combining? (1mk)
- Anode and cathode are separated by steal gauze diaphragm
- Anode and cathode are separated by steal gauze diaphragm
- Write an ionic equation for the reaction in which chlorine gas is formed. (1mk)
Cl-(aq) → Cl2(g) + 2e-
- What material is the anode made of? Give a reason (2mks)
- In the Downs process above a certain salt is added to lower the melting point of sodium chloride from about 800ºc to about 600ºc.
- Name the salt that is added. (1mk)
- Calcium chloride
- Calcium chloride
- State why it is necessary to lower the temperature. (1mk)
- To make the process less expensive by reducing electricity cost.
- To make the process less expensive by reducing electricity cost.
- Name the salt that is added. (1mk)
- Explain why aqueous sodium chloride is not suitable as an electrolyte for the manufacture of sodium in the Downs process. (2mks)
- Presence of H+ which will be preferentially be discharged.
- Presence of H+ which will be preferentially be discharged.
- Sodium metal reacts with air to form two oxides. Give the formulae of the oxides. (1mk)
- Na2O and Na2O2
- Below is a simplified diagram of the Down’s cell used for the manufacture of sodium. Study it and answer the questions that follow.
-
- One mole of heptane was thermally cracked, two hydrocarbons Q and P were formed.
Q was an alkene molecule with three carbon atoms.- Give the molecular formula of.
- Q - C3H6 // CH2 CHCH3
- P - C4H10 // CH3 CH2 CH2 CH3
- Write the structural formula of Q. (1 mark)
- Name the compound formed when Q undergoes self addition reaction. (1 mark)
- Polypropene
- Polypropene
- State one disadvantage of using the product named in a(iii) above. (1 mark)
- Pollutes the environment (as it is non-biodegradable)
- Pollutes the environment (as it is non-biodegradable)
- Cracking can also be achieved using less amount of heat in the presence of a catalyst. Name one catalyst that is often used. (1 mark)
- Alumina // Al2O3 or Silica // SiO2
- Alumina // Al2O3 or Silica // SiO2
- Give the molecular formula of.
- An organic compound J has the following percentage by mass, carbon 64.86%, hydrogen 13.51% and the rest is oxygen. The relative molecular mass of the compound is 74. C = 12, O = 16, H = 1)
- Work out the molecular formula of compound J. (3 marks)
Molecular formular =C4H10OCarbon Hydrogen Oxygen % 64.86 13.51 100-78.37=21.63 Moles 64.86/12=5.405 13.51/1=13.51 21.63/16=1.352 Moles 5.405/1.352=4 13.51/1.352=10 1.352/1.352=1 Empirical
formulaC4H10O (C4H10O)n =74 74n=74
n=1 - To which homologous series does compound J belong? (1 mark)
- Alkanols // Alcohols
- Alkanols // Alcohols
- Write a balanced chemical equation for the reaction that occurs when compound J reacts with sodium metal. (1 mark)
- 2C4H10O(l) + 2Na(s) 2C4H9ONa(aq) + H2(g)
- 2C4H10O(l) + 2Na(s) 2C4H9ONa(aq) + H2(g)
- Name the type of reaction indicated in b(iii) above. (1 mark)
- Displacement
- Displacement
- Name the organic compound formed when J reacts with excess acidified potassium managanate (VII). (1 mark)
- Butanoic acid
- Butanoic acid
- Work out the molecular formula of compound J. (3 marks)
- One mole of heptane was thermally cracked, two hydrocarbons Q and P were formed.
- 2.5g of a pure metal carbonate MCO3 was reacted with excess 2M nitric (V) acid. The volume of carbon (IV) oxide evolved was measured and recorded at 10 second intervals. The results were recorded as shown in the table below.
Volume of gas (cm³) 0 90 150 210 280 340 390 450 480 480 480 Time (seconds) 0 10 20 30 40 50 60 70 80 90 100 -
- On the grid provided, plot a graph of volume (vertical axis) against time – Label it curve A. (3 marks)
- Plotting – All points correctly plotted = (1mk)
9 points correctly plotted = (½mk)
<9 = (0mk)
Axes + scale – Maximum = (1mk)
Curve – Should be smooth = (1mk)
- Plotting – All points correctly plotted = (1mk)
- From your graph determine the rate of reaction between 25 seconds and 40 seconds. (2 marks)
- To be marked consequentially from students graph.
Penalize ½mk for wrong or missing units.
- To be marked consequentially from students graph.
- On the same axes, sketch a curve that would be obtained if the same experiment was repeated using excess 1M nitric (V) acid. Label it curve B. (1 mark)
- Curve B to be on the right of curve A and levelling at 480cm³ but later than A.
- Curve B to be on the right of curve A and levelling at 480cm³ but later than A.
- Give that carbon (IV) oxide was measured at room temperature and pressure, work out the relative atomic mass of metal M. (Molar gas volume at r.t.p = 24dm³, C = 12, O = 16). (3 marks)
- MCO3(s) + 2HNO3(aq) M(NO3)2(aq) + H2O(l) + CO2(g)
Moles of CO2 produced = 0.02
Moles of MCO3 = 0.02
0.02 mol MCO3 = 2.5g
1 mol (molar mass)
M + 12 + 48 = 125
M = 125 – 60 = 65
- MCO3(s) + 2HNO3(aq) M(NO3)2(aq) + H2O(l) + CO2(g)
- On the grid provided, plot a graph of volume (vertical axis) against time – Label it curve A. (3 marks)
- When bromine is dissolved in water the equilibrium shown below is established.
State and explain the observation that would be made if aqueous sodium hydroxide was added to the equilibrium mixture. (2 marks)- The yellow colour fades // Decolourisation of the mixture. Adding sodium hydroxide lowers the concentration of H+ ions. This makes equilibrium to shift to the right i.e. forward reaction is favoured lowering concentration of yellow Br2 molecules.
-
-
- Use the standard electrode potentials for elements A, B, C, D and F given below to answer the questions that follow.
- Which element is likely to be hydrogen? Give a reason for your answer. (2 marks)
- C // C2 Reject C+
It has an Eθ of zero // Being used as the standard electrode.
Reject – It has no e.m.f.
- C // C2 Reject C+
- What is Eθ of the strongest reducing agent? (1 mark)
- -290V Reject A or A2+
- -290V Reject A or A2+
- Calculate the e.m.f of the cell that would be formed when half cells of B and D are combined. (1 mark)
- Ecell = Ered – Eox
= +0.34 - -2.38
= +2.72V
- Ecell = Ered – Eox
- Which element is likely to be hydrogen? Give a reason for your answer. (2 marks)
- Aqueous copper (II) sulphate was electrolysed using the set up below.
- When the switch was closed a gas was produced only at electrode B. Which electrode is the anode? (1 mark)
- B
- Write the half equation for the reaction occurring at electrode B. (1 mark)
- 4OH-(aq) → 2H2O(l) + O2(g) + 4e-
- 4OH-(aq) → 2H2O(l) + O2(g) + 4e-
- What happens to the PH of the electrolyte during electrolysis? Explain. (2marks)
- Becomes acidic // PH lowers, reduces H+ ions remain in solution as OH- ions
are discharged.
- Becomes acidic // PH lowers, reduces H+ ions remain in solution as OH- ions
- If carbon electrodes were replaced with copper electrodes in the cell above, write the equation of the reaction that would occur at the anode. (1 mark)
- Cu(s)→Cu2+(aq)+2e-
- Cu(s)→Cu2+(aq)+2e-
- When the switch was closed a gas was produced only at electrode B. Which electrode is the anode? (1 mark)
- During electrolysis of aqueous copper (II) sulphate using copper electrodes a current of 0.2 amperes was passed through the cell for 5 hours. Determine the change in mass of the cathode that occurred as a result of the electrolysis process. (Cu = 64, IF = 96500 coulombs). (3 marks)
- Q = It
= 0.2 x 5 x 60 x 60
= 360ºC
Cu2+ + 2e- Cu
64g→ 2 x 9650ºC
y →36ºC
y = 36º x 64 = 1.194g
2 x 9650º
The cathode increased in mass by 1.194g.
- Q = It
- Use the standard electrode potentials for elements A, B, C, D and F given below to answer the questions that follow.
-
- State Hess’s law. (1 mark)
- Energy change in converting reactants A and B to products C and D is the same regardless of the route by which the chemical change occurs provided that the initial and final conditions are the same.
- Energy change in converting reactants A and B to products C and D is the same regardless of the route by which the chemical change occurs provided that the initial and final conditions are the same.
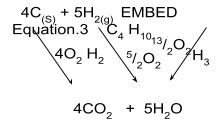
- Use the following information to answer the questions that follow:
C(S) + O2(g) → CO2(g) ΔH = -393KJ/mol
H2(g) + ½O2(g) → H2O(g) ΔH = -296KJ/mol
C4 H10 + 13/2O2(g) →4CO2(g)+ 5H2O ΔH = -2877KJ/mol- Draw an energy cycle diagram relating heat of formation and combustion of butane. (2 marks)
- Calculate the heat of formation of butane. (3 marks)
- ΔH1 - ΔH3 = ΔH2
ΔH1 = 4(-393) + 5(-286)
= -1572 – 1430
ΔH2 = -3002 - -2877 = -125KJ/mol
- ΔH1 - ΔH3 = ΔH2
- Draw an energy cycle diagram relating heat of formation and combustion of butane. (2 marks)
- Distinguish between hydration energy and lattice energy. (2 marks)
- Hydration energy is the enthalpy change when gaseous ions are hydrated by water.
- Lattice energy is the enthalpy change that occurs when one mole of a crystal structure is formed from its gaseous ions.
- The diagram below shows an energy level diagram for the formation of magnesium chloride. Study it and answer the questions that follow.
- State the enthalpy changes represented by
- (½ mark)- Heat of solution
- (½ mark)- Lattice energy
- (½ mark) - Hydration energy
- What is the relationship between ΔHA, ΔHBA and ΔHC. (½ mark)
ΔHA =ΔHB + ΔHC
(OR any other appropriate form)
- State the enthalpy changes represented by
-
- Define heat value of a fuel. (1 mark)
- Heat value of a fuel is the amount of heat energy produced when a unit mass of a fuel is completely burnt in oxygen.
- Heat value of a fuel is the amount of heat energy produced when a unit mass of a fuel is completely burnt in oxygen.
- Give two reasons why wood and charcoal are chosen for domestic heating. (2 marks)
- Environmentally friendly.
- Easy to transport and store.
- High calorific value.
- Readily available.
- Cheap.
- Non-poisonous.
- Burns slowly. Any two @ (1mk)
- Define heat value of a fuel. (1 mark)
- State Hess’s law. (1 mark)
- The flow chart below shows the large scale manufacture of ammonia gas and some ammonium compounds. Study it and answer the questions that follow.
- What are the sources of the following raw materials?
- Hydrogen gas. (1 mark) -
- Natural gas – e.g. Methane
- Crude oil
- Electrolysis of acidified water or brine
- Nitrogen gas. (1 mark) - Fractional distillation of liquid air.
- Hydrogen gas. (1 mark) -
- What optimum conditions are needed during the manufacture of ammonia in the
- Compressor. (1 mark) - Pressure of 200 – 500 atm.
- Catalytic chamber. (1 mark) - Temperature of 400 - 500°C.
Finely divided iron.
- Why should the gas be passed through the compressor. (1 mark)
- To compress the gases to high pressure which favour high yield of ammonia.
- Write an equation for the reaction that occurs in Step 1. (1 mark)
- 2NH3(g) + H2SO4(aq) (NH4)2SO4(aq)
- Write the formula of the compound B. (1 mark)
- NH4NO3
- Calculate the percentage of nitrogen in compound A. (2 marks)
- (NH4)2SO4 = 132
% of N
- (NH4)2SO4 = 132
- What observation would be made if compound C was added to a sample suspected to contain copper (II) ions dropwise then in excess? (2 marks)
- A pale blue precipitate on addition of a few drops precipitate dissolves in excess of C to form a deep blue solution.
- What are the sources of the following raw materials?
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Form 4 End Term 1 Exams 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students