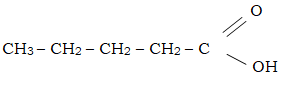Instructions
- Answer All questions in the spaces provided.
-
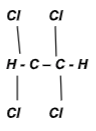
- Name the following organic compounds.
- CH3CH2CH(Br) C(Cl)(Br) CH3 (1mk)
-
- CH2CHCH2CH(Br)CH3. (1mk)
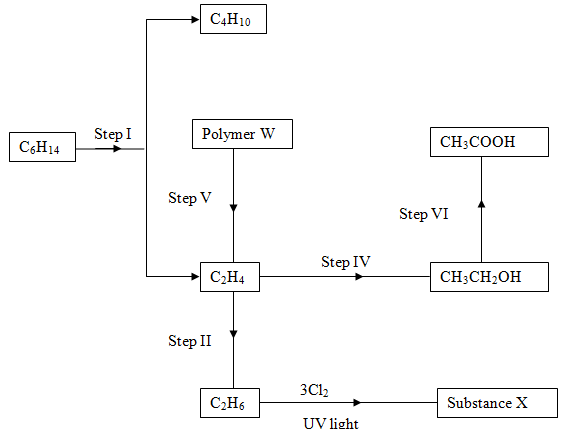
- Study the flow chart below and answer the questions that follow.
- Name;
- The processes that occur in steps marked I, IV and VI. (1 ½ mks)
- The reagent and conditions in step II. (1 ½ mks)
- Draw the structural formula of substance X, givethe name of the substance. (2mks)
- Name;
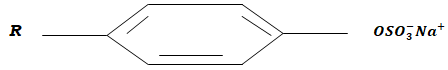
- The diagram below shows a structure of a cleansing agent.
- Name the cleansing agent above. (1mk)
- State the type of cleansing agent above. (1mk)
- Name the material added to the cleansing agent in order to improve its cleaning property. (1mk)
- Name the following organic compounds.
-
- The table below shows some properties of substances V, W, X and Z. Study them and answer the questions that follow. Letters do not represent the actual symbols of the substances.
Elements Solubility in water Boiling point (°C) Electrical conductivity Solid Molten V Insoluble 2955 Good Good W Soluble 1413 Poor Good X Insoluble −90 Poor Poor Z Insoluble 4827 Poor Poor - Which of the substances is likely, to have giant atomic structure? Explain. (2mks)
- Identify the particles responsible for conduction of electricity in V in solid and in molten states.
- Solid state ;…………………………………………………………. (1mk)
- Molten state : ……………………………………………………..
- Which substance has electrovalent bond? Explain. (2mks)
- Which substance is a gas at room temperature? (1mk)
- The table below shows some properties of halogens. Use is to answer the questions that follow.
Halogen Atomic radius (nM) Appearance Boiling point Fluorine 0.064 Pale-yellow gas −188 Chlorine 0.094 Greenish-yellow gas −35 Bromine 0.114 Brown liquid 59 Iodine 0.133 Shiny dark solid 184 - State and explain the trend in boiling points down the group. (2mks)
- State what would be observed when bromine water is added to potassium iodide solution. (1mk)
- Give a reason why iodine sublimes. (1mk)
- The table below shows some properties of substances V, W, X and Z. Study them and answer the questions that follow. Letters do not represent the actual symbols of the substances.
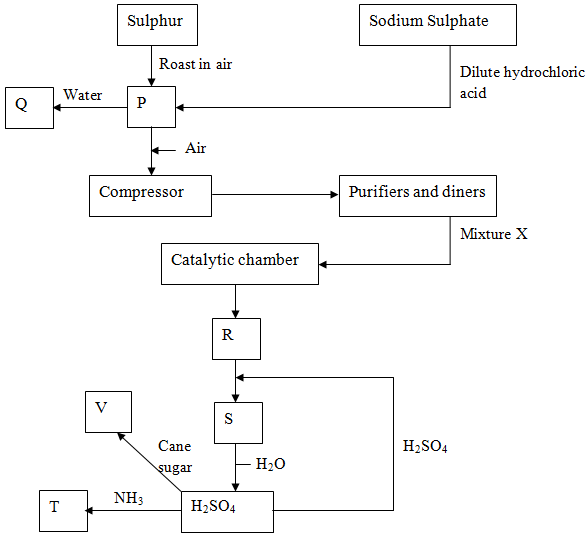
- Below is a simplified diagram for the manufacture of sulphuric (VI) acid in large scale.
- Name the substances P, Q, R, S, T and V. (3mks)
-
- What is the use of the compressor? (1mk)
- What is removed in the purification chamber? (1mk)
-
- State any two specific conditions for the formation of R. (1mk)
- Write the chemical formula of the catalyst used in the catalytic chamber. (1mk)
- Write an equation for the formation of gas R in the catalytic chamber. (1mk)
- Describe a chemical test to confirm the presence of P. (1mk)
- One of the uses of sulphuric (VI) acid is ‘pickling’ metals. What does the term ‘pickling’ mean? (1mk)
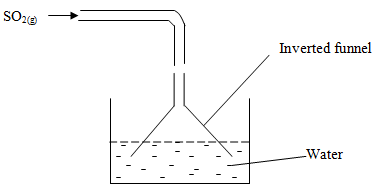
- The following diagram represents the method of preparing sulphur (IV) oxide solution.
- Why is an inverted funnel used? (1mk)
- Explain the observation made when sulphur (IV) oxide gas is mixed with universal indicator. (1mk)
- State and explain what would be observed if conc. H2SO4 is added to cane sugar leading to formation of substance U. (1mk)
- How can the pollution of the atmosphere be minimized in contact process? (1mk)
- A form four student wanted to find the proportion by volume of one of the main constitution of air. A sample of air was passed through two wash bottles, the first containing aqueous sodium hydroxide and the second containing concentrated sulphuric (VI) acid and was then collected in a gas syringe.
- Why was the air passed through:
- Aqueous sodium hydroxide. ½ mk)
- Concentrated sulphuric (VI) acid. ½ mk
- The volume of the air collected in the syringe was 90cm3. This was passed several times over hot copper powder until no further contraction of volume took place. After cooling to the original temperature, the volume was found to have reduced to 73.2 cm3.
- State and explain the observation made at the end of the experiment. (2mks)
- Calculate the percentage of the gas used up in the process. (2mks)
-
- Name the main gas remaining in the syringe. (1mk)
- is the main gas named in (i) above pure or not? Explain. (2mks)
- In another different experiment, the following results were obtained in a direct weighing.
Mass of flask + fittings = 184.257g
Mass of flask + fittings + gas P = 188.942g
Mass of flask + fittings + water = 988.560g
The volumes were measured at 23.0oC and 733mmHg pressure. Calculate the relative molecular mass of gas P. (Molar gas volume = 22.4 litres) (5mks)
- Why was the air passed through:
-
- Use the standard electrode potentials given below to answer the questions that follow.
Reaction Eθ value
Ag+(aq) + e− Ag(s) +0.80V
Cu2+(aq) + 2e− Cu(s) +0.34V
Pb2+(aq) + 2e− Pb(s) −0.13V
Zn2+(aq) + 2e− Zn(s) −0.76V- Select two half-cells which when combined give the largest workable cell. (1mk)
- With a reason, select the strongest oxidizing agent. (1mk)
- Draw a well labeled diagram for the electrochemical cell formed by combining copper and silver half cells. (3mks)
- Calculate the e.m.f of the cell formed when copper half-cell and lead half-cell are combined. (2mks)
- The following set-up was used to electrolyze copper (II) chloride solution using graphite electrodes.
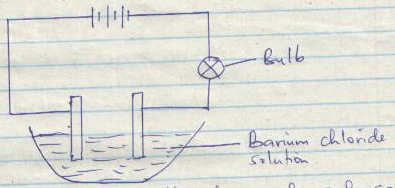
- On the diagram, label the anode and cathode. (1mk)
- State the observation made in the solution as the experiment progresses. Explain. (2mks)
- Write the ionic equation for the reaction at the anode. (1mk)
- Use the standard electrode potentials given below to answer the questions that follow.
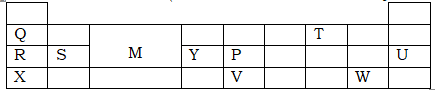
- The grid below represents part of the periodic table. Study it and answer the questions that follow. (The letters are not actual symbols of the elements)
- Select an element in period 3 which has the shortest atomic radius. Give a reason for your answer. (2mks)
- An element Z has the electronic structure 2.8.6. On the grid above, indicate the position of element Z. (1mk)
- What name is given to elements that belong to:
- Group I. (1mk)
- Region M. (1mk)
- Write an equation for the reaction of element Y with oxygen gas. (1mk)
- Elements S and W react to form a compound. In what forms will the compound formed conduct electricity? (1mk)
- Explain the following:
- Atomic radius of R is greater than that of Q. (1mk)
- The melting and boiling points of an oxide of Q are higher than those of oxide of T. (2mks)
- When 3 litres of chlorine gas were completely reacted with element S, 11.875g of the product were formed. Determine the relative atomic mass of element S. (R.A.M of Cl=35.5, molar gas volume = 24litres) (3mks)
- An experiment was carried out to determine the solubility of potassium nitrate and the following results were obtained.
Temperature 0 10 15 30 40 50 60 Mass in 100g of water 10 20 25 45 63 58 106 - Plot a graph of mass of potassium nitrate against temperature. (3mks)
- From the graph work out the mass of KNO3 that would crystallize if a solution containing 70g of KNO3 per 100g of water was cooled from 45°C to 25°C. (2mks)
- Explain what would happen if 100g of KNO3 was put in cold water and heated to 50°C. (2mks)

MARKING SCHEME
-
- Name the following organic compounds.
- CH3CH2CH(Br) C(Cl)(Br) CH3 (1mk)
- 2,3 – Dibromo – 2- chloropentane.
-
- Butanoic acid
- CH2CHCH2CH(Br) CH3. (1mk)
- 4-Bromopent-1-ene
- CH3CH2CH(Br) C(Cl)(Br) CH3 (1mk)
- Study the flow chart below and answer the questions that follow.
- Name;
- The processes that occur in steps marked I, IV and VI. (1 ½ mks)
- I – Cracking
- II- Hydrolysisi
- III- Oxidation
- The reagent and conditions in step II. (1 ½ mks)
- Reagent – hydrogen
- Conditions – temp 150 -250°C, nickel catalyst
- The processes that occur in steps marked I, IV and VI. (1 ½ mks)
- Draw the structural formula of substance X, give the name of the substance. (2mks)
- Name;
- The diagram below shows a structure of a cleansing agent.
- Name the cleansing agent above. (1mk)
- Sodium alkylbenzenesulphonate
- State the type of cleansing agent above. (1mk)
- Soapless detergent
- Name the material added to the cleansing agent in order to improve its cleaning property. (1mk)
- Tetraoxophosphate materials
- Name the cleansing agent above. (1mk)
- Name the following organic compounds.
-
- The table below shows some properties of substances V, W, X and Z. Study them and answer the questions that follow. Letters do not represent the actual symbols of the substances.
- Which of the substances is likely, to have giant atomic structure? Explain. (2mks)
- Z – It has very high boiling point and poor conductor in both states.
- Identify the particles responsible for conduction of electricity in V in solid and in molten states.
- Solid state ;…delocalized electrons………………. (1mk)
- Molten state : …delocalized electrons……………
- Which substance has electrovalent bond? Explain. (2mks)
- W – conducts electricity in molten and not in solid form/state
- Which substance is a gas at room temperature? (1mk)
- X
- Which of the substances is likely, to have giant atomic structure? Explain. (2mks)
- The table below shows some properties of halogens. Use is to answer the questions that follow.
- State and explain the trend in boiling points down the group. (2mks)
- b.p increases down the group due to increase in the strength of van der waals forces down the group.
- State what would be observed when bromine water is added to potassium iodide solution. (1mk)
- Dark brown solution is formed.
- Give a reason why iodine sublimes. (1mk)
- It has negligible temperature range in liquid state.
- State and explain the trend in boiling points down the group. (2mks)
- The table below shows some properties of substances V, W, X and Z. Study them and answer the questions that follow. Letters do not represent the actual symbols of the substances.
- Below is a simplified diagram for the manufacture of sulphuric (VI) acid in large scale.
- Name the substances P, Q, R, S, T and V. (3mks)
- P – sulphur (IV) Oxide
- Q – Sulphuric (IV) acid
- R – Sulphur (VI) oxide
- S – oleum
- T – Ammonium sulphate
- V – carbon
-
- What is the use of the compressor? (1mk)
- Increase pressure which increases the yield due to increased collisions of molecules.
- What is removed in the purification chamber? (1mk)
- Dust
- Water
- What is the use of the compressor? (1mk)
-
- State any two specific conditions for the formation of R. (1mk)
- Temp 450°C
- Pressure 2-3 atm
- Write the chemical formula of the catalyst used in the catalytic chamber. (1mk)
- V2O5
- Write an equation for the formation of gas R in the catalytic chamber. (1mk)
450°c
2SO2(g) + O2(g)2SO3(g)
V2O5
- State any two specific conditions for the formation of R. (1mk)
- Describe a chemical test to confirm the presence of P. (1mk)
- Mix a sample of P with Cr2O72−/ or MnO4-/H+.Cr2O72−/H+. Cr2O72−/H+turns green while MnO4- is decolourised.
- One of the uses of sulphuric (VI) acid is ‘pickling’ metals. What does the term ‘pickling’ mean? (1mk)
- Removal of oxide coating from the metal.
- The following diagram represents the method of preparing sulphur (IV) oxide solution.
- Why is an inverted funnel used? (1mk)
- To prevent sucking back of water due to high solubility of SO2 in water.
- Explain the observation made when sulphur (IV) oxide gas is mixed with universal indicator. (1mk)
- It turns red, since SO2 is acidic
- Why is an inverted funnel used? (1mk)
- State and explain what would be observed if conc. H2SO4 is added to cane sugar leading to formation of substance U. (1mk)
- the white crystals turn black due to formation of carbon.
- How can the pollution of the atmosphere be minimized in contact process? (1mk)
- SO2(g) emitted in the process is neutralized by Ca(OH)2 in chimenys through scrubbing.
- Name the substances P, Q, R, S, T and V. (3mks)
- A form four student wanted to find the proportion by volume of one of the main constitution of air. A sample of air was passed through two wash bottles, the first containing aqueous sodium hydroxide and the second containing concentrated sulphuric (VI) acid and was then collected in a gas syringe.
- Why was the air passed through:
- Aqueous sodium hydroxide. ½ mk)
- To remove carbon(IV) oxide gas .
- Concentrated sulphuric (VI) acid. ½ mk
- To dry the air
- Aqueous sodium hydroxide. ½ mk)
- The volume of the air collected in the syringe was 90cm3. This was passed several times over hot copper powder until no further contraction of volume took place. After cooling to the original temperature, the volume was found to have reduced to 73.2 cm3.
- State and explain the observation made at the end of the experiment. (2mks)
- Black solid is formed. √1 Volume of the gas reduced because oxygen present combined with copper to form CuO (black) √1
- Calculate the percentage of the gas used up in the process. (2mks)
Volume = 90 – 73.6 = 16.8cm3 √ ½
% of air used = 16.8 ×100% √ ½
90
= 18.67% √1
- State and explain the observation made at the end of the experiment. (2mks)
-
- Name the main gas remaining in the syringe. (1mk)
- Nitrogen √1
- is the main gas named in (i) above pure or not? Explain. (2mks)
- Impure √1 since it contains noble gases e.g argon and neon.
- Name the main gas remaining in the syringe. (1mk)
- In another different experiment, the following results were obtained in a direct weighing.
Mass of flask + fittings = 184.257g
Mass of flask + fittings + gas P = 188.942g
Mass of flask + fittings + water = 988.560g
The volumes were measured at 23.0°C and 733mmHg pressure. Calculate the relative molecular mass of gas P. (Molar gas volume = 22.4 litres) (5mks)
Mass of gas P = 188.942 – 184.257 = 4.685g √ ½
Volume of gas P = 988.560 – 184.257 = 804.303 cm3
P1 = 733 P2 = 760
V1 = 804.303 V2 = ?
T1 = 296 T2 = 273K
V2 = P1C1T2 = (733×804.303×273) = 715.45cm3
T1P2 296×760
4.685 g → 715.45cm3
? → 22400cm3
22400 × 4.685 =146.68g √1
715.45
- Why was the air passed through:
-
- Use the standard electrode potentials given below to answer the questions that follow.
Reaction Eθ value
Ag+(aq) + e− Ag(s) +0.80V
Cu2+(aq) + 2e− Cu(s) +0.34V
Pb2+(aq) + 2e− Pb(s) −0.13V
Zn2+(aq) + 2e− Zn(s) −0.76V- Select two half-cells which when combined give the largest workable cell. (1mk)
Zn(s)2+ |Zn(aq) ||Ag+(aq) | Ag(s) √1 accept Zn2+ and Ag+ - With a reason, select the strongest oxidizing agent. (1mk)
- Ag+. Has the most positive Eθ √1
- Draw a well labeled diagram for the electrochemical cell formed by combining copper and silver half cells. (3mks)
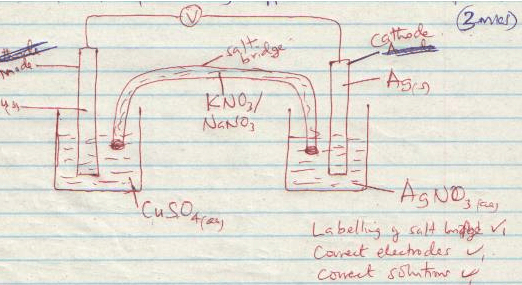
- Calculate the e.m.f of the cell formed when copper half-cell and lead half-cell are combined. (2mks)
E.m/f = Eθred − Eθoxide
= +0.34V−(−0.13V)
= 0.34 + 0.13
= +0.47V
- Select two half-cells which when combined give the largest workable cell. (1mk)
- The following set-up was used to electrolyze copper (II) chloride solution using graphite electrodes.
- On the diagram, label the anode and cathode. (1mk)
- State the observation made in the solution as the experiment progresses. Explain. (2mks)
- Blue colour of CuCl2 fades √1 More Cu2+ ions are deposited as Cu(s) √1
- Write the ionic equation for the reaction at the anode. (1mk)
2Cl−(aq) → Cl2(g) + 2e−
- Use the standard electrode potentials given below to answer the questions that follow.
- The grid below represents part of the periodic table. Study it and answer the questions that follow. (The letters are not actual symbols of the elements)
- Select an element in period 3 which has the shortest atomic radius. Give a reason for your answer. (2mks)
- U – has more protons (18) which strongly attract the outermost energy level closer to the nucleus reducing the radius. √1
- An element Z has the electronic structure 2.8.6. On the grid above, indicate the position of element Z. (1mk)
- Group VI and period 3
- What name is given to elements that belong to:
- Group I. (1mk)
- Alkali metals
- Region M. (1mk)
- Transition element
- Group I. (1mk)
- Write an equation for the reaction of element Y with oxygen gas. (1mk)
4Y(s) + 3O2(g) → 2Y2O3(s) √1 - Elements S and W react to form a compound. In what forms will the compound formed conduct electricity? (1mk)
- Aqueous and molten states
- Explain the following:
- Atomic radius of R is greater than that of Q. (1mk)
- R has 3 energy levels hence large atomic radius while Q has 2.
- The melting and boiling points of an oxide of Q are higher than those of oxide of T. (2mks)
- Oxide of Q forms giant ionic structure that need more energy to break while oxide of T forms simple molecular structures with weak van der waals forces that are easy to break .
- Atomic radius of R is greater than that of Q. (1mk)
- When 3 litres of chlorine gas were completely reacted with element S, 11.875g of the product were formed. Determine the relative atomic mass of element S. (R.A.M of Cl=35.5, molar gas volume = 24litres) (3mks)
Moles of Cl2 = 3/24 = 0.125moles
S + Cl2 → SCl2
1 : 1 : 1
Moles of SCl2 = 0.125 moles
Molar mass of SCl2 = 11.875 = 95
0.125
S + 71 = 95
S = 95 – 71
= 24
- Select an element in period 3 which has the shortest atomic radius. Give a reason for your answer. (2mks)
- An experiment was carried out to determine the solubility of potassium nitrate and the following results were obtained.
- Plot a graph of mass of potassium nitrate against temperature. (3mks)
- Scale ½
- Axes ½
- Curve 1mk
- Plotting 1mk
- Scale ½
- From the graph work out the mass of KNO3 that would crystallize if a solution containing 70g of KNO3 per 100g of water was cooled from 45°C to 25°C. (2mks)
70g – 42 (±1g) = 28g ± 1g - Explain what would happen if 100g of KNO3 was put in cold water and heated to 50°C. (2mks)
- At 50°C, only 85g will be in solution hence 100 – 85 = 15g will remain undissolved.
- Plot a graph of mass of potassium nitrate against temperature. (3mks)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Form 4 End Term 1 Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students