- An oxide of element D has the formulae D2O3
- What is the oxidation number of D? (1mk)
- Write the formulae of the hydroxide of D? (1mk)
- The diagram below shows the bonding present in water molecules
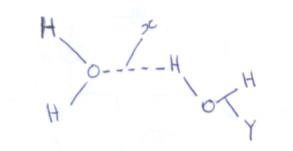
Name the type of bond indicated by- X (1mk)
- Y (1mk)
- When crystals of hydrated iron (II) sulphate, FeSO4.7H2O were left in the open air overnight, they changed to light green powder
- Explain the observations above (1mk)
- Name the process involved (1mk)
- Distinguish between hydrated salt and anhydrous salt (2mk)
- Study the set up below and answer the questions that follow
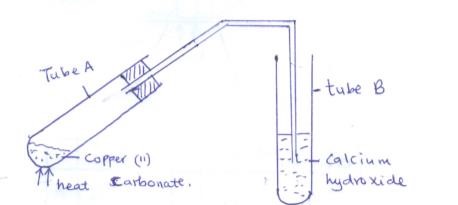
- State the observations made in
- Tube A (1mk)
- Tube B (1mk)
- Write a chemical equation for the reaction that took place in tube A. (1mk)
- State the observations made in
-
- State Boyles law (1mk)
- A sample of a gas occupies 800cm3 at 50oc and 760mmHg pressure. Determine the volume the gas would occupy at 0oC and 720mmHg pressure (3mk)
- Burning magnesium ribbon continues to burn in a gas jar of Carbon (IV) Oxide. Explain (3mk)
-
- State Grahams law of diffusion (1mk)
- The set up below was used to study the rate of diffusion of hydrogen and carbon (IV) Oxide
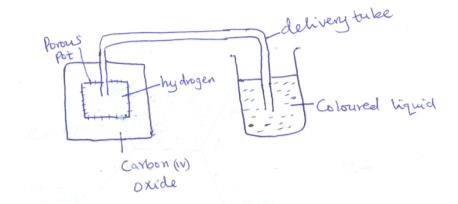
State and explain the observation which were made on the coloured liquid (3mk)
- A compound has the following percentage composition by mass: carbon 40%, hydrogen 6.7% and oxygen 53.3%. The relative formula mass, RFM of the compound is 180. Determine its;
- Empirical formula (C=12, H=1, O=16) (2mks)
- Molecular formula (2mk)
- Describe how you would obtain a pure sample of lead (II) carbonate from a solid mixture of lead (II) carbonate and sodium carbonate. (3mks)
-
- Define the term electrolyte (1mk)
- The set up below was used to electrolyse lead(II) bromide using graphite electrodes
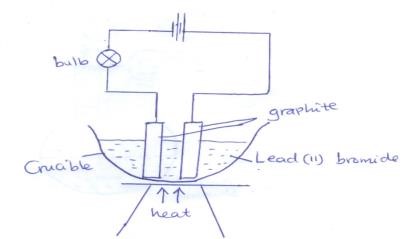
- Label the cathode on the diagram above (1mk)
- Write the ionic equation for the reaction that occurs at the
- Anode (1mk)
- Cathode (1mk)
- What observations would be made on the bulb if the source of heat was withdrawn? Explain. (2mks)
- A solution contains 4g of Sodium hydroxide in 250 cm3 of solution. Determine the molarity of the solution. (Na=23, O=16, H=1) (3mks)
- The grid below represents part of the periodic table. The letters are not the actual symbols of the elements.
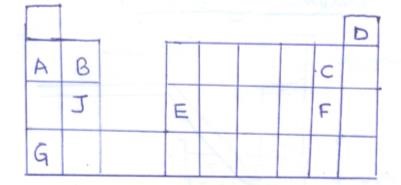
- Select the most reactive non metal (1mk)
- Select an element which
- Can form an ion of charge +2 (1mk)
- Has the least ionisation energy (1mk)
- Write the formula of the compound formed when E and F combine (1mk)
- What type of bond is formed when J and C combine? Explain (2mk)
- Compare the atomic radius of B and J. Give a reason for your answer (2mk)
- An atom of element W is denoted by 147 Indicate its position in the grid by writing letter W (1mk)
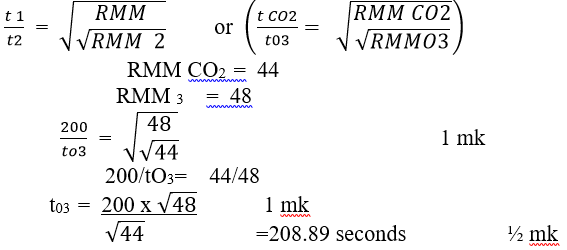
- A certain volume of carbon (IV) oxide takes 200 seconds to diffuse through a porous plug. How long would it take the same volume of Ozone, O3 to diffuse under the same conditions? ( C=12, O=16) (3mk)
- Two aqueous solutions of lead(II) nitrate and sodium chloride were mixed together
- Write the stoichiometric equation for the reaction that took place (1mk)
- Write the ionic equation for the reaction (1mk)
- The diagram below shows a charcoal ‘jiko’ with different regions
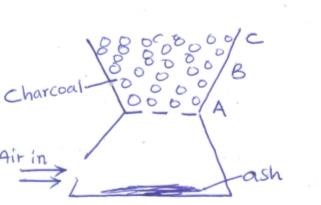
- Write an equation for the formation of the product in region B. (1mk)
- What observations are made in region C? Explain (2mk)
- Determine the number of molecules of oxygen gas contained in 11.2g of oxygen gas. ( O=16, Avogadro’s constant= 6.02 X 1023) (3mk)
- The table below shows the PH values of solutions A, B, C and D
SOLUTION
A
B
C
D
PH
6.5
13.0
8.0
2.0
- Which of the above solutions is likely to be dilute hydrochloric acid? (1mk)
- Name the type of reaction that takes place when B reacts with D (1mk)
- Which solution would be advisable for use by a patient with indigestion? (1mk)
- Complete the chemical equations below to show the decomposition of certain salts.
heat- NaHCO3(s) → (1mk)
heat - Zn(NO3)2(s) → (1mk)
- NaHCO3(s) → (1mk)
- Zinc metal was reacted with 200cm3 of 0.1 M hydrochloric acid according to the following equations
Zn (s) + 2HCl (aq) → ZnCl2(aq) +H2 (g)
Calculate the:- Number of moles of hydrochloric acid used (2mk)
- Volume of hydrogen gas produced at s.t.p. (molar gas volume at s.t.p=22.4 litres) (2mk)
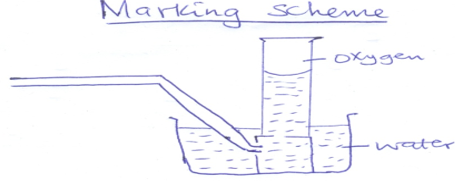
- The set up below was used to prepare oxygen in the laboratory
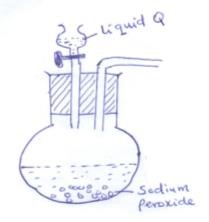
- Complete the diagram above to show how oxygen is collected (2mk)
- Identify the liquid Q (1mk)
- State one commercial use of oxygen (1mk)
- Study the table below and answer the questions that follow
Substance
Melting
point (0c)
Boiling
Point (0c)
Electrical conductivity
In solid
In liquid
U
1083
2595
good
good
V
801
1413
Poor
good
W
5
80
poor
poor
X
-115
-84
poor
poor
Y
3550
4827
poor
poor
- Which substance is likely to have the following structures
- Which substance is likely to be a metal? (1mk)
- Simple molecular (1mk)
- Giant ionic (1mk)
- 25cm3 of 0.1M potassium hydroxide solution was pipetted into a conical flask and titrated with dilute sulphuric (VI) acid. The results were tabulated as shown below.
I
II
III
Final burette reading (cm3)
24.1
48.1
24.4
Initial burette reading (cm3)
0.0
24.1
0.0
Volume of acid used (cm3)
24.1
24.0
24.4
- Calculate the average volume of the acid used (1mk)
- Calculate the number of moles of potassium hydroxide used (2mk)
- Determine the concentration of sulphuric (VI) acid used in moles per litre. (3mk)
FORM 3 CHEMISTRY
MARKING SCHEME
-
- +3 1 mk
- D(OH) 3 1 mk
-
- Hydrogen bonding 1mk
- Covalent bond 1 mk
-
- The crystals lost the water of crystallisation
- Efflorescence
- Hydrated salt-contains water of crystallisation. 1 mk
Anhydrous salt- does not contain water of crystallisation. 1 mk -
-
- green solid changes to black 1 mk
- White precipitate formed 1 mk
- CuCO3(s) → CuO(s) + CO2 (g)
-
-
- The volume of a fixed mass of gas is inversely proportional to its pressure at constant temperature.
- P1V1 = P2V2 ½ mk
T1 T2
T1 = 50+273= 323K)
T2 = 0+273=273K) ½mk
V2 =760x800x273
323x720 1 mk
= 713.73cm3 1 mk
- The heat (1mk) of burning magnesium decomposes CO2 into carbon and oxygen 1mk
The oxygen formed supports the burning of magnesium. -
- The rate of diffusion of a gas is inversely proportional to the square root of its density at constant temperature and pressure
- Level of coloured liquid rose in the delivery tube.
This is because H2 being less dense/lighter than CO2 diffuses faster out of the porous pot.
-
- C H O
Mole 40/12 6.7/1 53.3/16 ½mk
= 3.333 = 6.7 = 3.331 ½ mk
Ratio 1 2 1 ½ mk
E.F = CH2O ½ mk - (CH2O) n= 180
30n = 180
n = 6 1 mk
M.F = C6H12O6 1 mk
- C H O
- Add water to the mixture and stir ½ mk
Na2CO3 dissolves while PbCO3 does not ½ mk
Filter to obtain PbCO3 as residue 1 mk
Wash residue with distilled water and dry between filter papers. 1 mk -
- Is a substance that allows electric current to pass and is decomposed by it.
-
- Left –hand-side electrode
-
- 2Br-(l) → Br2(g) + 2e-
- Pb2+(l) + 2e- → Pb(s)
- Bulb goes off. (1mk) Lead (II) bromide becomes solid (1/2 mk) hence no free and mobile ions ½ mk
- RMM NaOH=23+16+1=40 ½ mk
1 mole of NaOH = 40g
? = 4g
1x4
40 ½ mk = 0.1 moles ½ mk
0.1 moles = 250cm3
? = 1000cm3
0.1x1000 1 mk
250
= 0.4M ½ mk
-
- C 1mk
-
- B or J 1 mk
- G 1 mk
- EF3 (reject F3E)
- Ionic / electrovalent bond 1 mk
Formed when J loses electrons and C gains the electrons. 1 mk
(reject: becauseJ is a metal and C a non metal ) - J has larger atomic radius than B (1mk)( or B has smaller atomic radius than J)
J has more energy levels than B 1mk - Group V and period 2 in the periodic table.
-
-
- Pb(NO3)2 (aq) + 2NaCl (aq) → PbCl2(s) + 2NaNO3(aq)
- Pb2+(aq) + 2Cl- → PbCl2(s)
- Pb(NO3)2 (aq) + 2NaCl (aq) → PbCl2(s) + 2NaNO3(aq)
-
- CO2(g )+ C(s) → 2CO(g)
- blue flame 1 mk
The carbon (II) Oxide / CO produced burns in air. 1 mk
- CO2(g )+ C(s) → 2CO(g)
- RMM O2=32
1 mole of O2 = 32g
? = 11.2g
1x11.2
32 1 mk
= 0.35 moles. ½ mk
1 mole = 6.02x1023 molecules
0.35 moles= 0.35x6.02 x 1023 1 mk
= 2.107x1023 molecules. ½ mk -
- D 1 mk
- Neutralisation reaction 1mk
- C 1 mk
-
- 2NaHCO3(s) → Na2CO3(s) + CO2(g )+ H2O
- 2Zn(NO3)2(s) → 2ZnO(s) + 4NO2(g) + O2(g)
-
- 0.1 moles= 1000cm3
= 200cm3
0.1x200
1000
=0.02 moles -
HCl : H2
1 mole -----------22.4 litre
Mole ratio 2 : 1
0.01 moles ------- 0.01 x 22.4 1 mk
0.02 : 0.01 moles
= 0.224 litres ½ mk
(Or 224 cm3)
- 0.1 moles= 1000cm3
-
-
NB: method 1 mk
Working 1 mk - Water / H2O
-
- Welding and cutting metals.
- By patients with breathing problems.
- By deep sea divers.
- By mountain climbers.
-
-
- Substance U
-
- X
- V
-
- 1+24.0 = 24.05 cm3
2 - 0.1 moles of KOH = 1000cm3
? = 25cm3
0.1x25
1000
= 0.0025 moles - 2KOH(aq) + H2SO4 (aq) → K2SO4(aq) + 2H2O(l)
Mole ratio 2 : 1
0.0025 : 0.0025 = 0.00125
2
0.00125 moles = 24.05 cm3
? = 1000cm3
0.00125x1000
24.05
= 0.052M ½ mk
- 1+24.0 = 24.05 cm3
Download CHEMISTRY - FORM 3 END TERM 1 EXAMS 2020.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students