When a solid B was heated in a test-tube, it gave off two gases. The two gases were separated by passing them through a plug of glass wool in a test-tube as shown below.
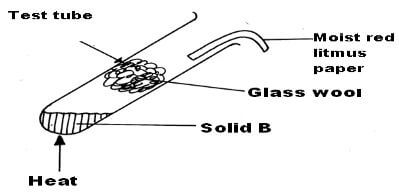
The first gas which evolved turned moist red litmus paper to blue. Later the other gas involved turned the litmus back to red.
- Identify solid B
- Write the equation for the reaction that take place in the test tube