Use the standard electrode potentials for elements A, B, C, D and F given below to answer the questions that follow.
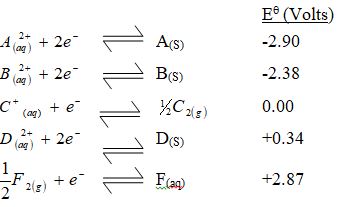
- Which element is likely to be hydrogen? Give a reason for your answer.
- What is Eθ of the strongest reducing agent?
- Calculate the e.m.f of the cell that would be formed when half cells of B and D are combined.