Chemistry Questions and Answers - Form 1 End Term 2 Exams 2023
Get the complete Chemistry Questions and Answers - Form 1 End Term 2 Exams 2023 PDF on WhatsApp by tapping on the button
- Answer All the questions
QUESTIONS
-
- What is a drug? (2mks)
- What is drug abuse? (2mks)
- One of the effects of drug abuse is hallucination. What does this term mean? (2mks)
- Name three frequently abused drugs? (3mks)
- Distinguish between a conductor and a non-conductor and give an example in each. (3mks)
- The diagram below shows the relationship between the physical states of matter. Study it and answer the questions that follow.
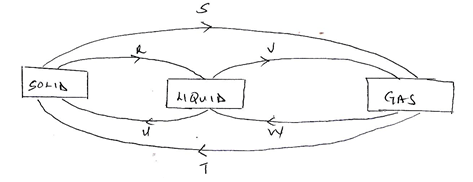
- Identify the process R,V,W and U (4mks)
- Name three substances which can undergo the process represented by process S and T. (3mks)
- The table below shows liquids that are miscible and those that are immiscible
Liquid L3 L4
L1 Miscible Miscible
L2 Miscible immiscible
Use the information given to answer the questions that follow.- Name the method that can be used to separate L1 and L3 from a mixture of the two. (1mk)
- Draw and name an apparatus that can be used to separate a mixture of L2 and L4. ( 3mks)
- Give two reasons why most Laboratory apparatus are made of glass. (2mks)
- Name three sources of heat beside Bunsen burner in the laboratory. (3mks)
-
- Draw a labeled diagram of a non-luminous flame produced by the Bunsen burner. (4mks)
- State two reasons why a non-luminous flame is preferred for heating. (2mks)
- After use a non-luminous flame should be put off or adjusted to a luminous flame. Explain. (2mks)
- Name three apparatus that are used to measure accurate volume of liquids. ( 3mks)
- Distinguish between an element and a compound and give an example of each. (3mks)
- By use of a diagram between a residue and a filtrate. (2mks)
- Name the method you would use to separate the following mixtures.
- Sand and ammonium chloride. (1mk)
- Oil and Water. (1mk)
- Kerosene and crude oil (1mk)
- Salt and water. (1mk)
- Describe how you would separate a mixture of salt, sand and iodine into different components. (3mks)
-
- State the functions of the following apparatus as used in the laboratory.
- Spatula (1mk)
- Pine-clay triangle (1mk)
- Wire gauze (1mk)
- Draw and state the use of a deflagrating spoon. (3mks)
- State the functions of the following apparatus as used in the laboratory.
- State the two causes of accidents in a Chemistry laboratory. (2mks)
- Define the following terms
- Solvent extraction (2mks)
- Hydrated salt (2mks)
- Saturated Solution (2mks)
- State two functions of a fume cupboard as found in a chemistry laboratory. ( 2mks)
- Explain the differences between solid and gaseous states using the theoretical model of matter in terms of the Kinetic theory. (3mks)
- The diagram below represents a paper chromatogram for three brands of juices suspected to contain banned food colourings.
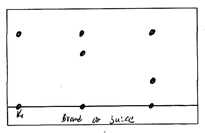
The results showed the presence or banned food colourings in L and M only.- On the same diagram
- Circle the spots which show the banned food colourings. (2mks)
- Show the solvent front. (2mks)
- State two factors that determine the position where the pigments are deposited in the paper chromatogram from the point of origin. (2mks)
- On the same diagram
- Classify the following processes as either chemical or Physical process type of change (3mks)
- Heating copper(ii) sulphate crystals
- obtaining Kerosene from crude oil
- Souring of milk.
- The figure below shows a heating curve of a certain pure solid.
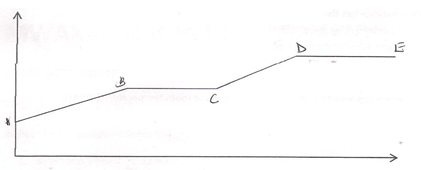
- What is happening at the stages represented by BC and CD (4mks)
- On the diagram draw a heating curve of an improve substance (2mks)
- Common table salt is contaminated with copper (ii) oxide. Explain how Pure sodium Chloride can be obtained from the mixture. ( 3mks)
- The table bellows gives information on some substances. Use it to answer the question that follows.
Substances Melting Point ºC Boliling point ºC Solubility in water
A - 177 78.5 Very Soluble
B -23 77 Insoluble
C -219 -183 slightly soluble
D -78 -33 Soluble- Which substance has the
- Lowest melting point (1mk)
- Highest boiling point (1mk)
- Which letters represents a substance that is a gas at room temperature? (2mks)
- Which is a liquid at room temperature and when mixed with water two layers would be formed. (1mk)
- Which substance dissolves in water and could be separated from the solution by fractional distillation. (2mks)
- Which substance has the
-
- Give the symbols of the following elements (3mks)
- Sodium
- Calcium
- Potassium
- Name the elements presents in the following compounds (2mks)
- Zinc sulphide
- Sodium oxide.
- Give the symbols of the following elements (3mks)
MARKING SCHEME
-
- A drug is any natural or man-made substances that in some way changes the way in which our body works.
- Drug abuse is the use of a drug for any purpose other than for which is intended.
- Hallucination - something that you think you can see or hear that is not really there, especially because of an illness or the effect of drugs.
- Any three
- Alcohol
- Tobacco
- Bhang
- Khat (miraa)
- Heroine
- Cocaine
- Conductors substances which allow electrical energy to flow through them. Example, metals.
Non-conductors are substances, which do not allow electrical energy to flow through them. For example; wood, papers, plastics. -
-
- R- melting
- V-evaporation
- W-condensation
- U-freezing
- Any three
- Iodine
- Solid carbon (IV) oxide
- Benzoic acid
- Ammonium chloride
- Alluminium (III) chloride
- Anhydrous Iron (III) chloride
-
-
- Fractional distillation
- Separating funnel

-
- Does not react with many reagents.
- Easy to observe the reaction (clear observation)
- Easy to clean
- Any three
- Spirit lamp
- Candle
- Gas stove
- kerosene stove
- Electric heater
-
-

- Very hot
Does not form soot. - Luminous flame is easily visible from a distance.
-
- Any three
- volumetric flasks
- syringes
- Pipettes
- Burettes
- measuring cylinder
- Elements are pure substances which cannot be split into simpler substances by any chemical means example sodium, potassium (award any correct element ) while a compound is a pure substance made up of two or more elements chemically combined.(example sodium chloride –award any correct compound)
-
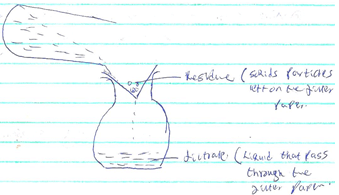
-
- Sublimation
- Separating funnel
- Fractional distillation
- Evaporation
- Place the mixture into a beaker and cover with a watch glass containing cold water. Heat the mixture gently and iodine solid will sublimate and settle on the cold watch glass. The remaining mixture adds water to it. Salt will dissolve but sand would not. Filter the mixture and sand is collected as the residue and salt as the filtrate. Evaporate water to collect salt as the solid.
-
-
- spatula – scooping solid substances from containers
- pipe clay-triangle - for supporting crucible during heating.
- Wire gauze- used for even distribution of heat when heating substances in beakers or flasks
- For holding substances being burnt.
-
-
- Ignorance
- Carelessness
-
- Solvent extractions- is a technique used to separate the components of a mixture based on their solubility in different solvents
- Hydrated salt - salt that contains water of crystallization
- Saturated solution - solution which no more solute can dissolve into it.
- Place where experiments which produce poisonous gases are carried out.
storing reagent which produce poisonous gases. -

-

In solid state ,the particles are closely packed together and can only vibrate within fixed positions hence shape and In gaseous state , the particles are far apart and free to more randomly in all directions hence no definite shape -
- Chemical change
- Physical change
- Chemical change
-
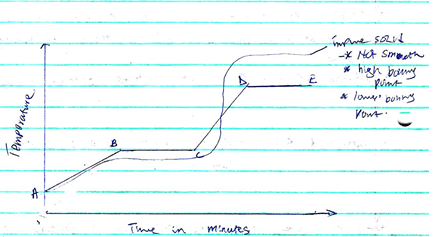
BC- The temperature remains constant until all the solid melts. Here, the heat supplied is used to weaken forces of attraction holding the particles of the solid together.
CD- Temperature rises steadily as the liquid of the pure substance absorbs heat energy .The heat supplied increases further, the kinetic energy of the particles causing them to move fast. - Add water to the mixture, stir and filter to obtain copper (II) oxide as a residue. Evaporate the filtrate to dryness to recover the sodium chloride crystals.
-
-
- C
- A
- C and D
- B
- A.
This is because they have different boiling points with water hence they can be separated by fractional distillation.
-
-
-
- Sodium Na
- Calcium Ca
- Potassium K
-
- Zinc Sulphide Zinc and Copper
- Sodium oxide sodium and Oxygen
-