Chemistry Paper 2 Questions and Answers - Form 3 End Term 1 Exams 2022
Get the complete Chemistry Paper 2 Questions and Answers - Form 3 End Term 1 Exams 2022 PDF on WhatsApp by tapping on the button
- The grid below represents part of the periodic table. Study it carefully and answer the questions that follow. (Letters do not represent the answer symbols of the elements.
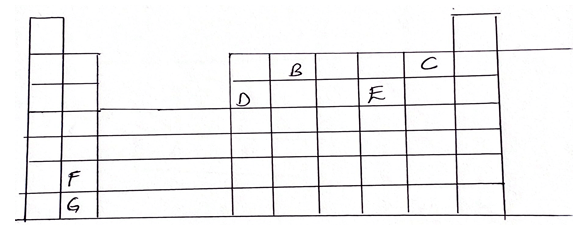
- Identify the family name to which element F and G belong. (1mks)
-
- Name the type of bond formed when c and F react (1mk)
- Name two other type of chemical bond. (1mk)
- Element D reacts with oxygen to form an Oxide of D. Write down the formula of the oxide. (1mk)
- What type of oxide is formed in (a) above. (1mk)
- Element X is in period 3 and forms an ion with the formula X3-
- Indicate its position on the grid. (1mk)
- Write down the formula of its chloride. (1mk)
-
- State two physical properties that copper and graphite share in common in relation to their structure and bonding (2mks)
- State two physical properties of simple molecular substances (3mks)
- Give one example of an element with a simple molecular structure which is a solid at room temperature. (1mk)
- What is an Isotope? (1mk)
- An element Q consists of 3 Isotopes of mass 28, 29 and 30, and percentage abundance of 92:2, 4.7 and 3:1, respectively. Determine the relative atomic mass of the element. (2mks)
- The flow chart below shows the Haber process use it to answer the questions that follow
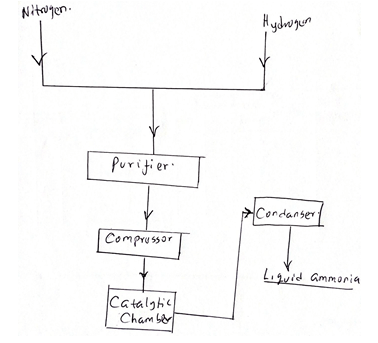
- Write the equation involves in production of ammonia. (1mk)
- Name three catalyst used in Haber process. (1mk)
- What is the role of the purifier? (1mk)
- State three function of the compressor. (1mk)
- Suggest a suitable source of hydrogen in Haber process. (1mk)
- State two uses of ammonia. (2mks)
- Availability of energy is one factor considered when setting ammonia plant. State other two factors (3mks)
- How many tonnes of nitric (iv) acid would be required to produce 24 tonnes of ammonium nitrate fertilizer (3mks) (H=1, N=14, O=16)
- Air was passed through several reagents as shown in the flow chart below.
- Name the major components of air. (1mk)
- Write down an equation for the reaction which takes place in the chamber with
- Concentrated sodium hydroxide (2mks)
- Excess heated copper turnings. (2mks)
- Excess heated magnesium powder. (2mks)
- Name one gas which escapes from the chamber containing magnesium powder. Give a reason for your answer. (2mks)
- Name the substance that was eliminated by electrostic precipitation. (1mk)
- Name a reagent that can be used in place of concentrated sodium hydroxide. (1mk)
- Name substance C. (1mk)
- State 2 uses of gas C. (2mks)
- The results below were obtained in an experiment conducted by form 3 students from Anestar Schools using magnesium
Mass of the crucible + lid = 19.52g
Mass of the crucible + lid+ magnesium ribbon = 20.36g
Mass of the crucible + lid + magnesium oxide= 20.92g-
- Using the results to find the percentage mass of magnesium and oxygen in magnesium oxide. (3mks)
- Determine the empirical formula of magnesium oxide (2mks)
- Sodium hydroxide pellets were accidentally mixed with Sodium Chloride 8.8g of the mixture was dissolved in water to make one litre of solution. 50cm3 of the solution was neutralized by20.0cm3 of 0.25M Sulphuric (Vi) acid.
- Write down an equation for the reaction that took place. (1mk)
- Calculate that:
- number of moles of the substances that reacted with sulphuric (vi) acid. (2mks)
- Number of moles of substances that reacted with sulphuric (VI) acid in the one litre solution (2mks)
- The percentage of sodium chloride in that mixture. (2mks)
-
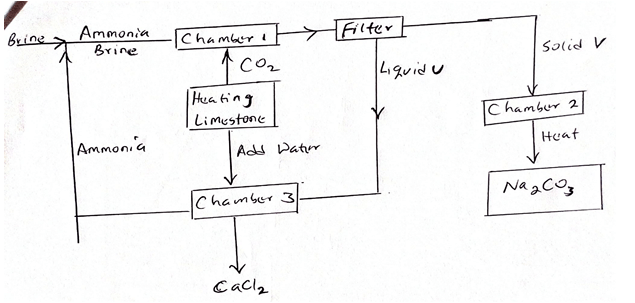
- The figure below shows the stages in the manufacture of sodium carbonate. Study it and answer the questions that follow.
-
- Name three starting materials in the manufacture of sodium carbonate. (3mks)
- Which substances are re cycled in this process (1mk)
- Identify the chambers in which the recycled substances’ are regenerated. (1mk)
- Name the substances U and V. (2mks)
- Give an equation for the reaction which occurs.
- In the reaction chamber. (2mks)
- When solid V is heated. (2mks)
- In the reaction chamber 3. (2mks)
- State one commercial use of Sodium Carbonate (1mk)
-
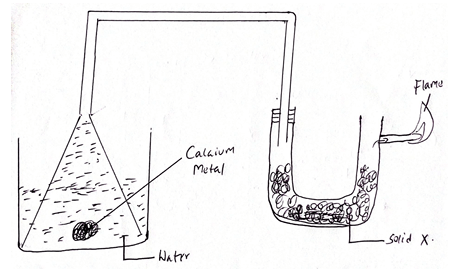
- The set up below was used to investigate the reaction between metals and water.
- Identify solid X and state its purpose. (2mks)
Solid X
Purpose - Write a chemical equation for the reaction that
- produces the flame (1mk)
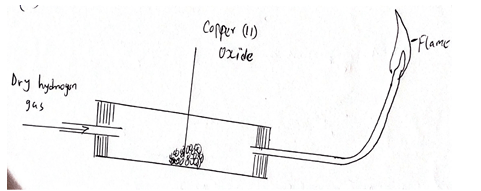
- The set up below was used to investigate the properties of hydrogen.
- On the diagram, indicate what would be done for the reaction to occur. (1mk)
- Hydrogen gas is allowed to pass through the tube for some time before it is lit. Explain. (1mk)
- Write an equation for the reaction that occurs in the combustion tube. (1mk)
- When the reaction is complete, hydrogen gas is passed through the apparatus until it cools down. Explain (2mks)
- What property of hydrogen is being investigated (1mk)
- What observation confirms the property stated in (V) above. (2mks)
- Why is Zinc Oxide not used to investigate this property of hydrogen gas (2mks)
- Identify solid X and state its purpose. (2mks)
MARKING SCHEME
-
- Alkaline earth metals
-
- Ionic bond
- Covalent bond
Metallic bond
- D2O3
- An amphoteric oxide
-
- X should be placed in the box or the left of E
- XCl3 or XCl5
-
- Both are coordinates of gg
Both have high melting points and boiling points due to their giant structures - low melting and boiling points
poor conductors of heat and electricity
most occurs in gaseous state - Iodine, sulphur or phosphorous
- A molecule that is partially charged, owing to differences in altercation of shared pair of electrons forming covalent bonds. It is a result of the difference in negativity
- 92.2 x 28 + 4.7 x 29 + 3.1 x 30
100 100 100
= 28.11
- Both are coordinates of gg
-
- N2(g) + 3H2(g) → 2NH3(g)
- Finely divided iron
- To remove impurities in the gases e.g CO2, water vapour and dust which may poison the catalyst
- To provide higher pressure, more ammonia is provided under high pressure
- Natural gas
Cracking of crude oil - Fertilizer
Nitric acid manufacturer
Stain removal
As a refrigirant - Availability of raw material
Availability of labour
Availability of transport - HNO = 1 + 14 + (16 x 3) = 63
NH4NO3 = (14 x 2) + (1 x 4) + (10 x 3) = 80
NH3(g) + HNO3(aq) → NH4NO3(aq)
63g 80g
80g of NH4NO3 require 63g of HNO3
24 000 000g x 63 = 18 900 000g
80 1 000 000
= 18.9 tonnes
-
- Nitrogen, oxygen, carbon(iv) oxide
-
- 2NaOH + CO2(g) → Na2CO3(aq) + H2O(I)
- 2CO(g) + O2(g) → 2CO(g)
- 3Mg(g) N2(g) → Mg3N2(s)
- Argon - It is an inert gas
- Dust particles
- Concentrated potassium hydroxide (KOH)
- Oxygen gas
- In hospital to aid patients with breathing difficulties
To burn fuel in rockets
Used to remove impurities during steel making
-
-
- Mass of magnesium = 20.36 - 19.52 = 0.849
Mass of oxygen = 20.92 - 20.36 = 0.56g
Mass of MgO = 20.92 - 19.52 = 1.40g
% of magnesium =(0.84) x 100 = 60%
1.40
% of mass of oxygen in MgO = (0.56 x 100) = 40%
1.40
= MgOElements Mg O Mass 0.84 0.56 Moles 0.84
24
0.0350.56
16
0.035Simple ratio 1 1
- Mass of magnesium = 20.36 - 19.52 = 0.849
-
- 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(I)
-
- Mole ratio of NaOH : H2SO4 = 2:1
Moles of NaOH = 2 x 0.005 = 0.01moles - If 50cm3 of NaOH = 0.01moles
1000cm3= x
(1000cm3 x 0.01 moles) = 0.02moles
50cm3
OR
(Ans in (i) x 1000) = 0.02moles
50 - Molar mass of NaOH = 23 + 1 + 16 = 40g
Mass of NaOH = 40 x 0.2 = 8g
Mass of NaCl = (0.8 x 100) = 9.0909%
0.8
- Mole ratio of NaOH : H2SO4 = 2:1
-
-
-
- Ammonia gas, calcium carbonate, brine(NaCl)
Concentrated sodium chloride and coke - Carbon(iv) oxide, ammonia gas and water
- Chamber 3
- U = ammonia chloride
V = sodium hydrogen carbonate
- Ammonia gas, calcium carbonate, brine(NaCl)
-
- NaCl(g) + NH3(g) + CO2(g) + H2O(g) → NaHCO3(g) + NH4Cl(aq)
- NaHCO3(g) → Na2CO3(g) + CU2(g) + H2O(I)
- Ca(OH)2(g) + 2NH4Cl(aq) → CaCl2(aq) + 2NH3(g)+ 2H2O(I)
- Manufacturer of glass
Softening of hard water
Manufacturer of soap
-
-
- Solid x - Anhydrous calcium chloride/Calcium oxide
Pupose - to dry the hydrogen gas -
- 2H2(g) + O2(g) → 2H2O(g)
-
- ..
- To drive away air to avoid explosion
- CuO(g) + H2(g) → Cu(g) + H2O(I)
- So as to avoid re-oxidation of the hot copper metal by oxygen in air
- Reducing property
- Black CuO turns to brown Cu solid
Colorless liquid formed - Zinc is above hydrogen in the relativity
Series, more reactive hence hydrogen cannot displace zinc
- Solid x - Anhydrous calcium chloride/Calcium oxide