The diagram below shows the energy changes that occur when sodium chloride dissolves in water. Study it and answer the questions that follow.
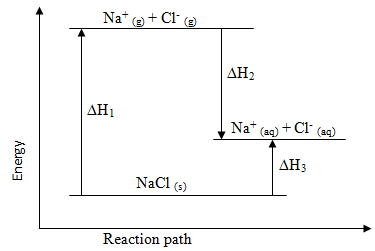
- What do ∆H1, ∆H2 and ∆H3 represent?
- Given that the lattice energy of NaCl (s) is -776 kJmol-1 and hydration energies of Na+(g) and Cl-(g) are -407 kJmol-1 and -364 kJmol-1 respectively. Calculate the heat of solution (∆Hsol) of 1 mole NaCl (s).