Below is a simplified diagram of the Down’s cell used for the manufacture of sodium. Study it and answer the questions that follow.
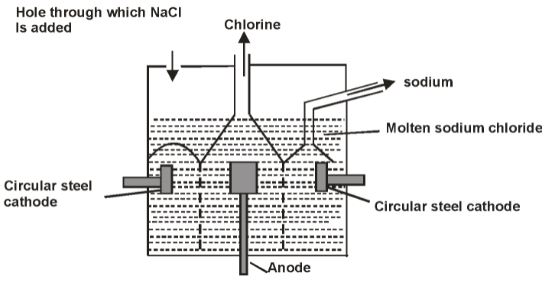
- What material is the anode made of? Give a reason
- What precautions are taken to prevent chlorine and sodium from re-combining?
- Write an ionic equation for the reaction in which chlorine gas is formed.
- In the Downs process above a certain salt is added to lower the melting point of sodium chloride from about 800ºc to about 600ºc.
- Name the salt that is added.
- State why it is necessary to lower the temperature.