Aqueous Copper (II) Sulphate was electrolyzed using the set up represented by the diagram below
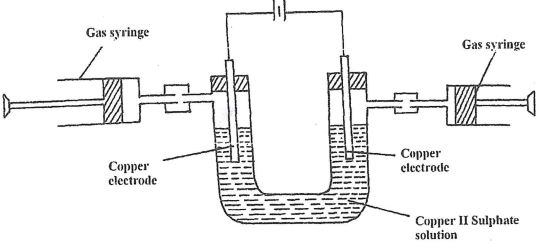
- After sometimes it was found that no gas was produced at both electrodes. Explain.
- Write an equation for the reaction at each electrode.
- Anode
- Cathode
- What happens to the colour of the electrolyte during electrolysis. Explain
- An iron spoon is to be electroplated with silver. Draw a labeled diagram of the set-up that could be used to represent the process.
- The following are half-cell equations for some elements. The letters do not represent actual symbols. Use the information to answer the questions that follow.
Half cell Eθ, v
M2+(aq)+ 2ē → M(s) +0.34
L2+(aq)+2ē → L(s) +0.84
K2+(aq)+ 2ē → K(s) -0.13
J2+(aq) + 2ē + J(s) -0.76- Select the two half cells that would produce the highest e.m.f of a cell.
- Calculate the e.m.f of the cell in d(1) above.
- Give the cell diagram notation for the cell in d(ii) above.