Study the flow chart below and answer the questions that follow
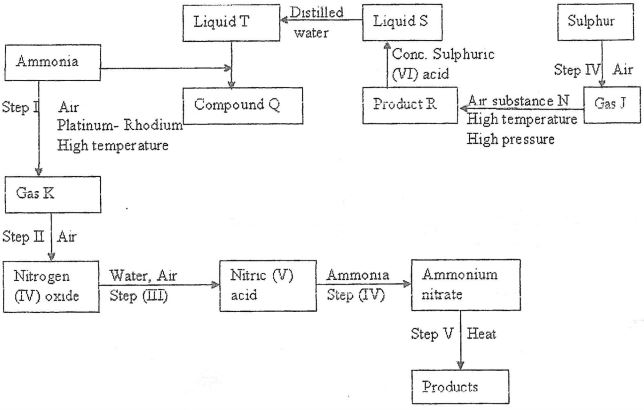
- Identify gas:
- J
- K
- Write the equation for the reaction that occurs in steps:
(V)
(VI) - Name substance N
- Write an equation for formation of R and explain why high pressure is required in converting J to R
- Give one commercial use of;
- Liquid T
- Compound Q
- Describe a chemical test that would be used to distinguish the anion in compound Q and the product in Step IV
- State and explain the observation made when concentrated sulphuric (VI) is added to crystals of copper (II) Sulphate in a beaker.