The grid below shows a part of the periodic table. The letters do not represent the actual symbols. Study it and answer the questions that follow.
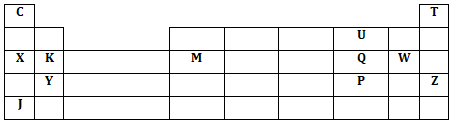
- Identify the elements in period 1
- With a reason, identify the element with the largest atomic radius
- Draw the atomic structure of element Q
- Write down the electronic configurations of elements Y and W
- Y- ……………………………………………………………………….
- W- ………………………………………………………………………
- Element G forms an ion G3- and its ionic configuration 2.8.8. indicate its position on the grid above
- Identify an element whose oxide reacts with both acids and alkalis
-
- Write down the chemical formular of the compound formed between elements K and
- Draw the bonding in the compound formed in (g) (i) above using dots (.) and crosses (x) to represent electrons
- Compare the atomic radius elements X and K. Explain