Hydrogen gas was prepared in the lab. Using the following set up
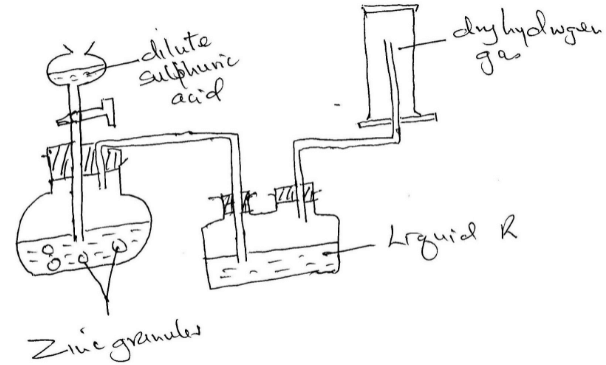
- Write an equation for the reaction taking place and balance it
- Name the method used to collect the gas and give a property of hydrogen that enables it to be collected through the method.
- Name liquid R and state its function in the set up
- Explain why it is not advisable to use sodium metal in place of zinc metal
- What will happen to the pH of the solution in the beaker after one day? Give an explanation.