Nitrogen(IV) oxide is prepared by heating lead(ll) nitrate.
- Write an equation for the reaction.
- At room temperature, nitrogen(IV) oxide exists as an equilibrium mixture with dinitrogen tetraoxide.
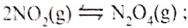 Δ is-ve
Δ is-ve
(brown) (pale yellow)
State the observation made when the mixture is placed in an ice-bath. Give a reason.