The grid below is part of the periodic table. Use it answer the questions that follow. (The letters are not the actual symbols of the elements).
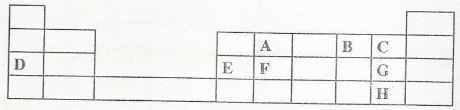
- Which is the most reactive non-metallic element shown in the table? Explain.
-
- Write the formula of the compound formed when element A reacts with element B.
- Name the bond type in the compound formed in b (i) above.
- What is the name given to the group of elements where C, G and H belong?
- The melting points of elements F and G are 1410 0C and -101 0C respectively. In terms of structure and bonding, explain why there is a large difference in the melting points of F and G. (2 marks)
- D forms two oxides. Write the formula of each of two oxides. (1 mark)
- J is an element that belongs to the 3rd period of the periodic table and is a member of the alkaline earth elements. Show the position of J in the grid.