- Give two differences between luminous and non-luminous flames. (2 marks)
Luminous
Non-luminous
- Bright yellow flame
- Sooty
- 4-regions
- Large and wavy
- Blue flame
- Non-sooty
- 3-region
- Short and steady
- How is the non-luminous flame produced? (1 mark)
- When the air hole is fully open.
- Give two differences between luminous and non-luminous flames. (2 marks)
- The apparatus below were used to separate a mixture of liquid A and B.
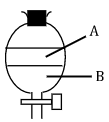
- State two properties of liquids that make it possible to separate using such apparatus. (2 marks)
- Immiscible
- Different densities - Give the name of the above apparatus. (1 mark)
- Separating funnel
- State two properties of liquids that make it possible to separate using such apparatus. (2 marks)
- Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice cream venders. (1 mark)
- Better coolant
- Sublims leaving no wetness - Name one piece apparatus used to measure volume of gases. (1 mark)
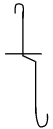
- Syringe - Draw a diagram of a deflagrating spoon. (1 mark)
- Explain why solid Carbon (IV) oxide is preferred over ordinary ice for use by ice cream venders. (1 mark)
- The table below shows the pH values of solutions P, R, Q and S.
Solution
P
R
Q
S
pH
2
7
6.5
13.5
- Which solution represent:
(i) Strong base - S (1 mark)
(ii) Weak acid - Q (1 mark) - Give an example of solution S. (1 mark)
- Sodium hydroxide/potassium hydroxide
- Which solution represent:
- 6.95g of hydrated iron (II) sulphate FeSO4. nH2O was dissolved in 250 cm3 solution resulting into a 0.1M solution. Determine the value of n. (3 marks) (Fe = 56, O = 16, S = 32, H = 1).
Moles of soln
If 0.1 moles are in 1000cm3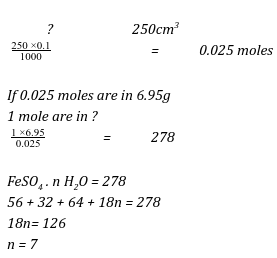
- Rusting leads to fast wearing out of farm tools and equipment as well as buildings.
- Give the chemical name of rust. (1 mark)
- Hydrated Iron (III) oxide - What two conditions accelerate rusting process? (2 marks)
- High temperatures
- Acidic conditions
- Salty conditions
- Give the chemical name of rust. (1 mark)
- Study the diagram below and answer the questions that follow.
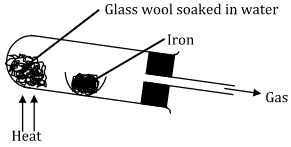
- Write an equation for the reaction that take place in the combustion tube. (1 mark)
- 3Fe(s) + 4H2O(g)------> Fe3O4(s) + 4H2(g) - Why would it not be advisable to use potassium in place of iron in the set-up? (1 mark)
- It is more reactive, hence reacts explosively with steam. - Glass wool should be heated before heating iron. Explain. (1 mark)
- So as to generate/evolve steam first
- Write an equation for the reaction that take place in the combustion tube. (1 mark)
- Name the following organic compounds.
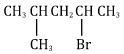
2-bromo-4-methyl pentane- HOCH2 - CHOH - CH2OH (1 mark)
Propen-1, 2, 3 - trio1
- Given
- CH3(CH2)16 COO- Na+
- CH3(CH2)6 CH(CH3)CH2 SO-3 Na+
a - Soapy (1 mark)
b - Soapless (1 mark)
- Name the following organic compounds.
- In terms of structure and bonding, explain the following.
- Graphite is used as a lubricant. (1 mark)
- The hexagonal layers are joined by weak van der wals forces which can slide over each other easily is the giant cordent structure. - Alluminium is better conductor of electricity then magnesium. (1 mark)
- Has more delocalized electrons in their giant metallic structure. - Water is a liquid at room temperature while hydrogen sulphide is a gas. (1 mark)
- Water has strong hydrogen bonds as compared to the weak van der wals forces in H2S in their molecular structure.
- Graphite is used as a lubricant. (1 mark)
- Define the term molar latent heat of fusion. (1 mark)
- Molar latent heat of fusion of a substance is the amount of heat absorbed when one mole of the solid is converted into the liquid state at constant temperature and pressure. - The molar heat of fusion of ice at O0C is 6kJ mol-1. Calculate the heat change when 36g of ice is converted to 36g of water at 100C. (3 marks) (SHC = 4.2-1g K-1, density = 1.0g/cm3, H = 1.0, O = 16.0)
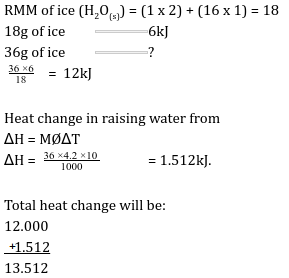
- Define the term molar latent heat of fusion. (1 mark)
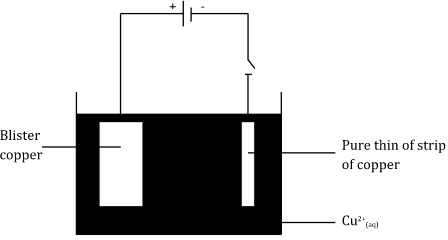
- Draw a well labeled diagram showing how blister copper is purified. (3 marks)
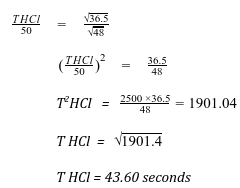
- Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the same amount of hydrogen Chloride (HCl) to diffuse through the same diaphragm under similar conditions? (H = 1.0, Cl = 35.5). (3 marks)
- Calculate the oxidation state of chromium in the ion Cr2 O2-. (1 mark)
2cr + -2 x 7 = -2
2cr - 14 = -2
2cr = +12
Cr = +6 - Using oxidation numbers, determine from the equation below the species which undergoes oxidation and reduction.
2FeCl2(aq) + Cl2(g) 2Fe Cl3(aq)
Oxidation - Fe2+ (Iron (II) ions) (1 mark)
Reduction - Chlorine (1 mark)
- Calculate the oxidation state of chromium in the ion Cr2 O2-. (1 mark)
- Given elements A, B and C with atomic numbers 11, 19 and 13 respectively.
- Compare the atomic radius of A and C. Explain. (2 marks)
- C is smaller than A. It has more protons which increases nuclear attraction. - Compare reactivity of A and B. (1 mark)
- B is more reactive than A.
- Compare the atomic radius of A and C. Explain. (2 marks)
- Haber process (the manufacture of ammonia gas) is given by the following equation.
N2(g) + 3H2(g) <======> 2NH3(g) ΔH = -92kJ mol-1.
State and explain the effect of:- Introducing some drops of water to the equilibrium. (1 mark)
- The equilibrium shifts to the right to replace the ammonia gas absorbed by water molecules. - Pumping nitrogen gas to the equilibrium mixture. (1 mark)
- The equilibrium shifts to the right to use up the nitrogen gas added. - Lowering the temperature of the reaction. (1 mark)
- Forward reaction if exothermic hence favoured by low temperatures. Therefore the equilibrium shifts to the right.
- Introducing some drops of water to the equilibrium. (1 mark)
- Elements P and Q have the following atomic numbers 19 and 8 respectively.
(i) Using dot (.) and cross(x) draw a diagram to show how the elements form bonds. (1 mark)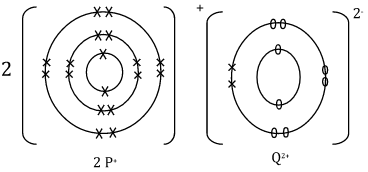
- Describe how sodium sulphate crystals can be prepared starting with 50cm3 of 2M sodium hydroxide and 1M sulphuric (VI) acid. (3 marks)
- Moles of NaOH = 0.1 moles
2NaOH(aq) + H2SO4(aq) --------> Na2SO4(aq) + 2 H2O(l)
Moles of H2SO4 required = 0.05 moles
Volume of H2SO4 required = 0.05 = 50cm3
- Measure 50cm3 of 1M H2SO4 and add it to 50cm3 of 2M NaOH(aq) in a beaker.
- Heat the mixture to concentrate the solution.
- Allow the concentrated solution to evaporate slowly to form crystals of sodium sulphate. - Write ionic equations to show how;
- (i) Excess ammonia solution reacts with a solution containing Copper II ions. (1 mark)
Cu(OH)2(s) + 4NH3(aq) ---------->[Cu(NH3)4]2+(aq) + 2OH-(aq)
(ii) Excess sodium hydroxide added to a solution containing Al3+ ions.
Al(OH)3(s) + OH-(aq)-------------> [Al(OH)4]-(aq) - Give the name of the following ion [Zn(NH3)4]2+ (1 mark)
- Tetramine zinc ions.
- (i) Excess ammonia solution reacts with a solution containing Copper II ions. (1 mark)
- Define electrolysis. (1 mark)
- Process of the decomposition of an electrolyte by passing electric current through it. - During the electrolysis of molten aluminium oxide, write the equations at the;
Anode - 6O2-(l) 3O2(g) + 12e- (1 mark)
Cathode - 4Al3+ + 12e- 4Al(s). (1 mark)
- Define electrolysis. (1 mark)
- Give any two differences between alpha and beta particles. (2 marks)
Alpha Beta Positively charged Very Charged Heavy Light - A radioactive isotope T decays by emitting three alpha particles to form what is the atomic number and mass number T?
Atomic number - 89 (1 mark)
Mass number - 226 (1 mark)
- Give any two differences between alpha and beta particles. (2 marks)
- Using acidified potassium dichromate (VI) solution, describe how you would differentiate between sulphur (IV) oxide and hydrogen sulphide. (2 marks)
- Bubble the gases into separate test tube containing acidified potassium dichromate (VI) solution; Both change potassium dichromate (VI) solution from orange to green, but a yellow solid overdue/deposited is formed with hydrogen sulphide no yellow deposit formed with sulphur (IV) oxide. - Identify the catalyst preffered in ......... Explain (2 marks)
- Platinum and vanadium (V) oxide.
- Vanadium (V) oxide is preferred because it is cheaper and less easily poisoned.
Ammoniated brine
- Using acidified potassium dichromate (VI) solution, describe how you would differentiate between sulphur (IV) oxide and hydrogen sulphide. (2 marks)
- Study the following part of the solvay process for the manufacture of sodium carbonate and answer the questions that follows:
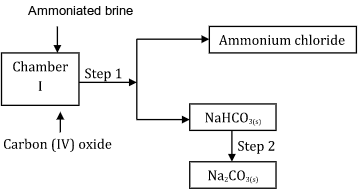
(i) State the main source of Carbon (IV) oxide in the process. (1 mark)
- (Heating) coke/calcium carbonate.
(ii) Write down the overall equation for the reaction in chamber I. (1 mark)
NaCl(aq) + NH3(g) + H2O(l) + CO2(g) -----------------> NaHCO3(s) + NH4Cl(aq)
(iii) Name process in step 1. (1 mark)
- Cooling
- The reaction is exothermic - The following equation involve hydrochloric acid.
MnO2(s) + 4HCl(aq)-----------------> MnCl2(aq) + 2H2O(l) + Cl2(g)- State the type of reaction taking place in the reaction. (1 mark)
............................................... - State two contrasting chemical properties of hydrogen and chlorine. (2 marks)
Hydrogen Chlorine Reducing agent Oxidising agent Does not bleach Bleaching agent
- State the type of reaction taking place in the reaction. (1 mark)
- An element O has 2 isotopes containing 90% and Isotope .
(i) What are isotopes? (1 mark)
- They are atoms of some elements with some atomic number but different mass number due to different number of neutrons.
(ii) Find the R.A.M of O. (2 marks)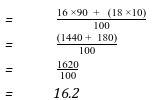
- An element O has 2 isotopes containing 90% and Isotope .
- When a hydrocarbon is completely burnt in oxygen 4.2g of Carbon (IV) oxide and 1.71g of water were formed.
- Determine the empirical formular of the hydrocarbon. (3 marks)
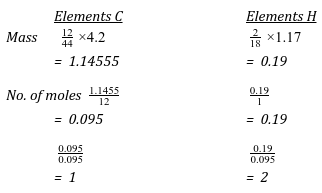
- Given that formula mass of compound above is 28. Find the molecular formular. (1 mark)
(CH2)n = 28
14n = 28
n = 2
C2H4
- Determine the empirical formular of the hydrocarbon. (3 marks)
- Name the two types of polymerization. (1 mark)
- Addition
- Substitution - Study the section of the polymer below and answer the questions that follow.
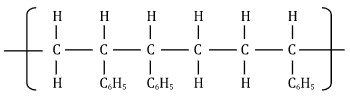
(i) Give the name of the polymer above. (1 mark)
- Polyphenylethene/ polyslyrene
- Name the two types of polymerization. (1 mark)
Download KASSU JOINT EVALUATION EXAMINATION CHEMISTRY Paper 1 with answers.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

