Instructions to Candidates
- Answer ALL the questions in the spaces provided below each question.
- You are NOT allowed to start working with the apparatus for the first 15 minutes of the 2¼ hours allowed for this paper. This time is to enable you to read the question paper and make sure you have all the apparatus and chemicals that you may need.
- All working MUST be clearly shown where necessary
- Mathematical tables and silent non-programmed electronic calculators may be used.
- You are provided with:
- Solution A – 2M Hydrochloric acid.
- Solution B – 0.2M sodium hydroxide solution.
- 6 pieces of 2cm length of magnesium ribbon
You are required to; - Determine the mass of magnesium ribbon that reacted with hydrochloric acid
Procedure I- Using a clean measuring cylinder, measure 50 cm3 of solution A into a 100 ml glass beaker.
- Cut the magnesium into six (6) pieces each of 2 cm long.
- Put one piece of magnesium ribbon into the solution A in the 100ml glass beaker and simultaneously start the stop watch.
- Record the time taken by magnesium ribbon to get completely finished in table I.
- Record procedure (iii) and (iv) using the same solution in procedure (i) adding each piece of magnesium ribbon up to the 6th piece and complete the table I below. Label the resultant solution as M and retain it for procedure II.
Table I
(5 marks)Magnesium ribbon Number 1st 2nd 3rd 4th 5th 6th Time taken (s) 1 (S−1)
time- Plot a graph of 1time (vertical axis) against the magnesium ribbon number. (3 marks)
- From the graph, determine the time that would be taken for 5 cm piece of the ribbon to get completely finished. (2 marks)
Procedure II
Transfer all the solution M from procedure I into a 250ml volumetric flask. Top up the flask to the mark with distilled water and shake. Label as solution N.- Fill the burette with solution N.
- Using a pipette filler, place 25cm3 of solution B in a 250ml conical flask. Add 2 drops of phenolphthalein indicator and titrate with solution N.
- Record your results in table II. Repeat the titration two more times to complete table II.
Table II
(4 marks)1 2 3 Final burette reading Initial burette reading Volume of Solution N used (cm³)
Calculate the:- average volume of solution N (1 mark)
- moles of sodium hydroxide, solution B used. (1 mark)
- moles of hydrochloric acid, in solution N used. (1 mark)
- moles of hydrochloric acid in 250cm3 of solution N. (1 mark)
- moles of hydrochloric acid in 50cm3 of solution A. (1 mark)
- moles of hydrochloric acid in solution A that reacted with all pieces of magnesium ribbon. (1 mark)
- mass of magnesium ribbon used in the reaction. (Mg = 24) (2 marks)
- You are provided with solid P. Carry out the tests below and record your observations and inferences in the spaces provided.
Place solid P in a clean boiling tube, add 10cm3 of distilled water and shake well. Use about 2cm3 portions of the mixture for the tests below.- Add aqueous ammonia dropwise until in excess.
Observations Inferences (1 mark) ( 1mark) - Add 2M sodium hydroxide solution dropwise until in excess.
Observations Inferences (1 mark) ( 1mark) - Add 4 drops of 2M sulphuric (VI) acid.
Observations Inferences (1 mark) ( 2marks) - Add 3 drops of lead (II) nitrate solution
Observations Inferences (½ mark) ( 1mark) - Add 3 drops of barium nitrate solution
Observations Inferences (½ mark) ( 1mark)
- Add aqueous ammonia dropwise until in excess.
- You are provided with an organic substance Q. Carry out the tests below and record your observations and inferences in the spaces provided.
- To about 2cm3 of substance Q in a test tube, add 2 drops of acidified potassium manganate (VII) solution.
Observations Inferences (1 mark) ( 1mark) - Place 3 drops of substance Q on a watch glass and ignite.
Observations Inferences (1 mark) ( 1mark) - To about 2cm3 of substance Q, add 2cm3 of ethanoic acid followed by 5 drops of 2M sulphuric (VI) acid and heat.
Observations Inferences (1 mark) ( 1mark) - To another 2cm3 of substance Q, add 2 drops of acidified potassium dichromate (VI) solution.
Observations Inferences (1 mark) ( 1mark)
- To about 2cm3 of substance Q in a test tube, add 2 drops of acidified potassium manganate (VII) solution.
CONFIDENTIAL
- Each candidate
- About 60 cm3 of solution A
- About 100cm3 of solution B
- One burette 0 – 50 ml
- One pipette 25.0ml and a pipette filler
- One Filter funnel
- 100ml glass beaker
- Watch glass
- 13cm long of Magnesium ribbon
- Two clean dry 250 ml conical flasks
- Five (5) clean and dry test tubes on a test tube rack
- One boiling tube
- 10ml measuring cylinder
- Stopwatch
- 5cm3 of ethanoic acid supplied in a stoppered test tube
- About 500cm3 of distilled water supplied in a wash bottle
- One 250ml volumetric flask
- One 50ml measuring cylinder or 100ml measuring cylinder
- Two (2) labels
- One metallic spatula
- One test tube holder
- A white tile
- A wooden splint
- About 0.5g of solid P supplied in a stoppered container
- About 10ml of substance Q supplied in a syringe
- A scarpel
- Access to:
- Phenolphthalein indicator supplied with a dropper
- Bunsen burner
- 2.0M aqueous ammonia supplied with a dropper
- 2.0M sodium hydroxide solution supplied with a dropper
- 2.0 M sulphuric (VI) acid
- Acidified potasium dichromate (VI) solution supplied with a dropper
- Acidified potassium manganate (VII) solution supplied with a dropper
- 0.5M Barium (II) nitrate solution supplied with a dropper
- 0.5M Lead (II) nitrate solution supplied with a dropper
- Preparation of solutions and solids
- Solution A (2.0M Hydrochloric acid) prepared by 172cm3 of concentrated hydrochloric acid diluted with distilled water to make a litre of solution.
- Solution B (0.2M Sodium Hydroxide) is prepared by dissolving 8.0g of sodium hydroxide in 700cm3 of distilled water and diluting it to one litre.
- Solid P is Aluminium sulphate
- Substance Q is Absolute Ethanol
- 13 cm long Magnesium ribbon
MARKING SCHEME
- You are provided with:
- Solution A – 2M Hydrochloric acid.
- Solution B – 0.2M sodium hydroxide solution.
- 6 pieces of 2cm length of magnesium ribbon
You are required to; - Determine the mass of magnesium ribbon that reacted with hydrochloric acid
Procedure I- Using a clean measuring cylinder, measure 50 cm3 of solution A into a 100 ml glass beaker.
- Cut the magnesium into six (6) pieces each of 2 cm long.
- Put one piece of magnesium ribbon into the solution A in the 100ml glass beaker and simultaneously start the stop watch.
- Record the time taken by magnesium ribbon to get completely finished in table I.
- Record procedure (iii) and (iv) using the same solution in procedure (i) adding each piece of magnesium ribbon up to the 6th piece and complete the table I below. Label the resultant solution as M and retain it for procedure II.
Table I
(5 marks)Magnesium ribbon Number 1st 2nd 3rd 4th 5th 6th Time taken (s) 35.62 40.00 47.62 55.56 66.69 83.41 1 (S−1)
time0.028 0.025 0.021 0.018 0.015 0.012
CT3
DP½
AC½
TR1- Plot a graph of 1time (vertical axis) against the magnesium ribbon number. (3 marks)
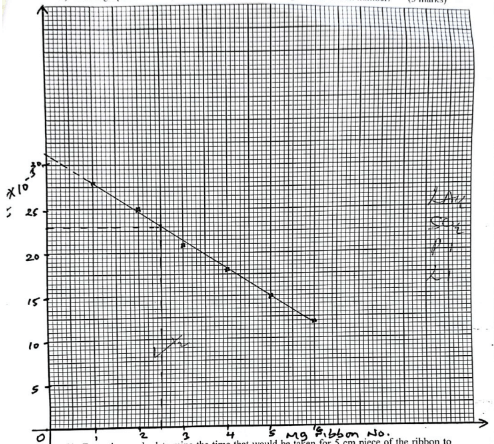
- From the graph, determine the time that would be taken for 5 cm piece of the ribbon to get completely finished. (2 marks)
1/t = 23 × 10−3
t = 1/23 × 103
= 43.48sec
showing ½✓
Reading ½✓
Working ½✓
Correct ans ½✓
Procedure II
Transfer all the solution M from procedure I into a 250ml volumetric flask. Top up the flask to the mark with distilled water and shake. Label as solution N.- Fill the burette with solution N.
- Using a pipette filler, place 25cm3 of solution B in a 250ml conical flask. Add 2 drops of phenolphthalein indicator and titrate with solution N.
- Record your results in table II. Repeat the titration two more times to complete table II.
Table II
(4 marks)1 2 3 Final burette reading 20.5 20.6 20.7 Initial burette reading 0.0 0.0 0.0 Volume of Solution N used (cm³) 20.5 20.6 20.7
CT1
DP1
AC1
PA1
FA1
Calculate the:- average volume of solution N (1 mark)
20.5 + 20.6 + 20.7 = 20.6
3 - moles of sodium hydroxide, solution B used. (1 mark)
= 0.2 × 25
1000
= 0.005 - moles of hydrochloric acid, in solution N used. (1 mark)
moles of HCl = moles of NaOH
= 0.005 - moles of hydrochloric acid in 250cm3 of solution N. (1 mark)
0.005 × 250 = 0.0607
20.6 - moles of hydrochloric acid in 50cm3 of solution A. (1 mark)
2 × 50 = 0.1
1000 - moles of hydrochloric acid in solution A that reacted with all pieces of magnesium ribbon. (1 mark)
= 0.1 − 0.0607
= 0.0393 - mass of magnesium ribbon used in the reaction. (Mg = 24) (2 marks)
Moles of Mg used
= 0.0393
2
= 0.01965
mass = 0.01965 × 24
= 0.4716g
- average volume of solution N (1 mark)
- Plot a graph of 1time (vertical axis) against the magnesium ribbon number. (3 marks)
- You are provided with solid P. Carry out the tests below and record your observations and inferences in the spaces provided.
Place solid P in a clean boiling tube, add 10cm3 of distilled water and shake well. Use about 2cm3 portions of the mixture for the tests below.- Add aqueous ammonia dropwise until in excess.
Observations Inferences White ppt
Insoluble in excess (1 mark)Mg2+, Al3+, Pb2+
Present Accept Zn2+ absent ( 1mark) - Add 2M sodium hydroxide solution dropwise until in excess.
Observations Inferences White ppt
Soluble in excess (1 mark)Al3+, Pb2+ Present ( 1mark) Penalise ½mk each for any contradictory ion to a max of 1mk - Add 4 drops of 2M sulphuric (VI) acid.
Observations Inferences No white ppt
No effervescence (1 mark)Al3+, Present
SO32−, CO32− absent ( 2marks) - Add 3 drops of lead (II) nitrate solution
Observations Inferences white ppt (½ mark) SO42−,Cl−/Br− present ( 1mark) - Add 3 drops of barium nitrate solution
Observations Inferences White ppt (½ mark) SO42− present ( 1mark) Penalise FULLY for any contradicting ion.
- Add aqueous ammonia dropwise until in excess.
- You are provided with an organic substance Q. Carry out the tests below and record your observations and inferences in the spaces provided.
- To about 2cm3 of substance Q in a test tube, add 2 drops of acidified potassium manganate (VII) solution.
Observations Inferences Purple KMnO4
Solution is decolourised (1 mark)( 1mark)
- Place 3 drops of substance Q on a watch glass and ignite.
Observations Inferences Almost colourless/pale blue flame (1 mark) ( 1mark)
- To about 2cm3 of substance Q, add 2cm3 of ethanoic acid followed by 5 drops of 2M sulphuric (VI) acid and heat.
Observations Inferences Pleasant/fruity smell
reject: Sweet smell (1 mark)R−OH present ( 1mark) - To another 2cm3 of substance Q, add 2 drops of acidified potassium dichromate (VI) solution.
Observations Inferences Orange K2Cr2O7 Solution changes to green (1 mark) R−OH present ( 1mark)
- To about 2cm3 of substance Q in a test tube, add 2 drops of acidified potassium manganate (VII) solution.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 3 Questions and Answers - Maranda High School Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
