The diagram below shows an experiment involving chlorine water.
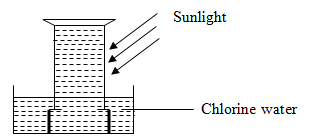
- Chlorine water is a mixture of two acids. Explain using a chemical equation.
- State and explain the observation that was made after 24 hours.
- Write an equation for the reaction that took place in (2) above.