Study the diagram below and answer the questions that follow:
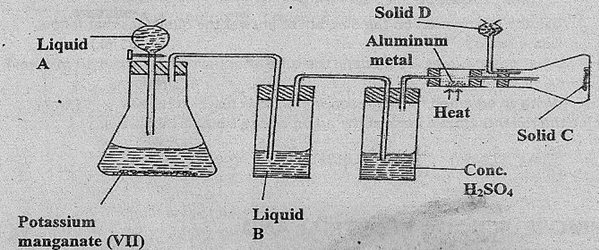
- Name Liquids A and B
- Solid D can be anhydrous calcium chloride. Suggest another suitable reagent that can be used in place of anhydrous calcium chloride.
- State the role of D suggested in (ii) above.
- write a balanced equation for the reaction in the conical flask .
- Explain why solid C collects further away from the heated aluminum metal.
- In the combustion tube above 0.675g of aluminum metal reacted completely with 1800cm3 of chlorine gas at room temperature. Determine the molecular formula of solid C given that its relative formula mass is 267. (Al = 27, Cl = 35.5, Molar gas volume at r.t.p. = 24.0 litres)