Below is a set of apparatus that was used to obtain a dry sample of sulphur(iv)oxide gas
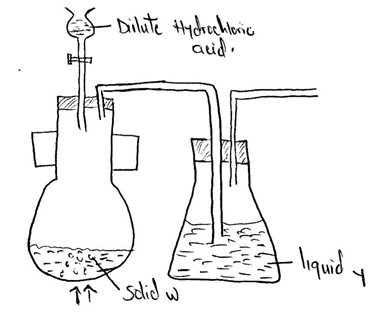
- Name;
- Solid W
- The apparatus containing dilute hydrochloric acid
- State the role of Liquid Y
- Complete the diagram to show how the gas could have been collected
- A sample of sulphur(iv)oxide gas was passed through freshly prepared iron(III)sulphate solution. State and explain the observation made
- 50cm3 of 2M Hydrochloric acid was used during the above experiment. Determine the volume of sulphur(iv)oxide gas produced at r.t.p (molar gas volume = 24dm3)