The diagram below shows the set up used by a student to prepare dry sulphur IV oxide gas. Study it carefully and answer the question that follows.
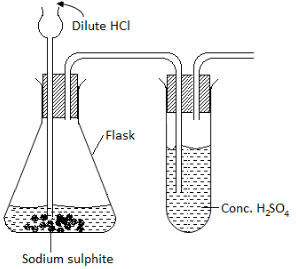
- Complete the diagram to show how to collect dry sulphur (IV) oxide.
- Write an equation for the reaction in the flask.
- Give another pair of chemical that could be used to prepare sulphur (IV) oxide.
- Sulphur IV oxide gas was bubbled through concentrated nitric (V) acid. State and explain the observations made.