The scheme below shows various reactions starting with hydrogen and nitrogen .study it carefully and answer the question that follows.
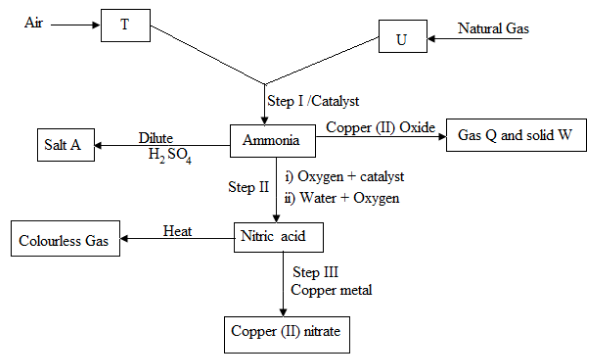
- Name the substances:
- T
- U
- A
- Q
- W
- C
- Name the catalyst which could be used in:
- Step I
- Step II
- Write equations for the reaction occurring in:
- Step I
- Step II (two equations)
- What property of ammonia gas is shown in its reaction with copper II oxide?